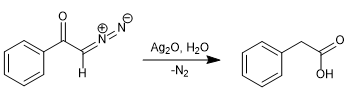
In 1902, Wolff observed that treating diazoacetophenone (α-diazoketone) with Ag2O/H2O produced a rearrangement that generated phenylacetic acid.
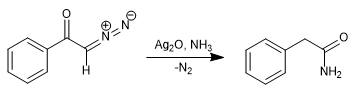
By replacing water with ammonia, phenylacetamide is obtained.
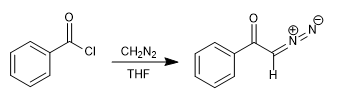
The α-diazoketones are obtained by reacting acid halides with two equivalents of diazomethane in ethereal solution (Arndt-Eistert reaction.
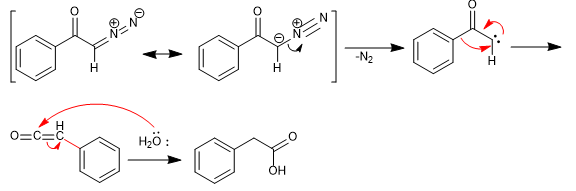
Mechanism:
The reaction proceeds through the formation of a carbene by loss of nitrogen. The carbene evolves by regrouping to ketene, which is attacked by water to give the corresponding carboxylic acid.