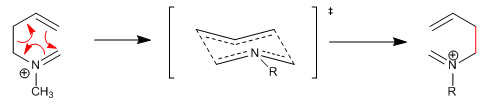
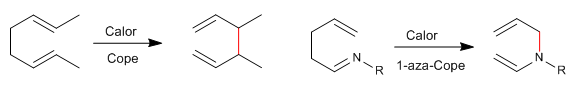
1,5-Dienes isomerize by sigmatropic-[3,3] rearrangements on heating. Reaction known as Cope rearrangement. The rearrangement of N-substituted 1,5-dienes is called an aza-Cope rearrangement. Depending on the position occupied by nitrogen we have: 1-aza-, 2-aza-, 3-aza-Cope. The 3-aza-Cope regrouping coincides with the aza-Claisen.
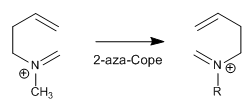
The reaction goes under milder conditions if the nitrogen is charged (ammonium salt)
The aza-Claisen reaction is a concerted process that proceeds through a six-membered transition state that assumes a chair arrangement with the substituents arranged nearly equatorially.