The hydrazones [3] are obtained by reaction of aldehydes or ketones [1] with hydrazine [2]. As in the case of imines and oximes, it requires pH=4. 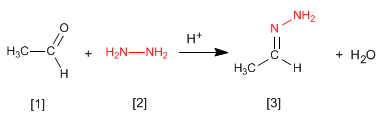
Although the mechanism is analogous to that of imine formation, we will discuss the steps again. 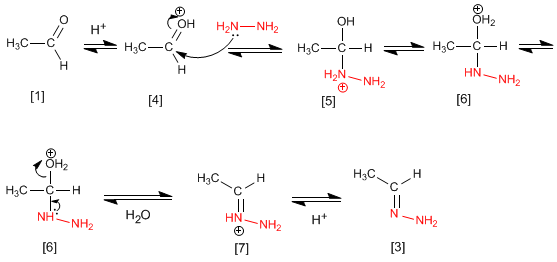
The ethanal [1] is protonated to form its conjugate acid [4]. The important polarity of the carbonyl carbon of favors hydrazine attack for forming the intermediate [5]. The compound exchanges a proton between nitrogen and oxygen, transforming the hydroxyl group into water (good leaving group). The intermediate [6] loses a water molecule becoming [7], the deprotonation of which gives the final hydrazone [3] .









