The reaction between 2-aminoethylbenzene and an alkanoyl halide in the presence of pyridine forms an amide. The amide is converted to the Vilsmeier electrophile by reaction with phosphorous oxytrichloride. The cyclization is produced by attack of the benzene to said electrophile. A final oxidation generates isoquinoline.
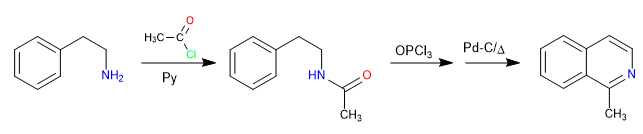
Mechanism:
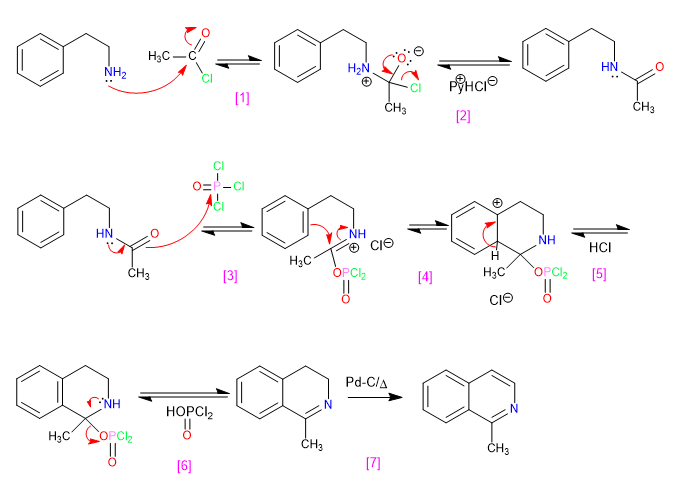
Nucleophilic addition to acid halide
Hydrogen Chloride Removal
Phosphorus oxytrichloride attack
Cyclization by attack of benzene on the electrophile
Recovery of aromaticity.
Elimination
Oxidation