Visible-Ultraviolet Spectroscopy
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 84314
Spectroscopy studies the interaction between electromagnetic radiation and matter. In this interaction, electromagnetic radiation can behave as a wave or as a particle, although no physical phenomenon has been observed in which both behaviors occur simultaneously.
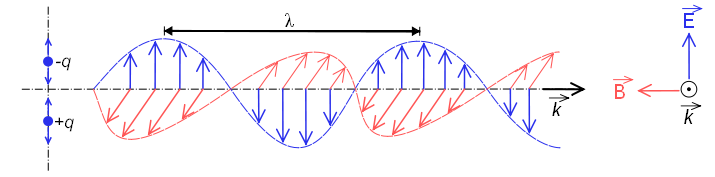
When it behaves as a wave, it is made up of an electric field and a magnetic field that oscillate perpendicular and propagate at the speed of light $c=300000\;km/s$
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 19445
There are three zones of the electromagnetic spectrum with special interest in the determination of chemical compounds:
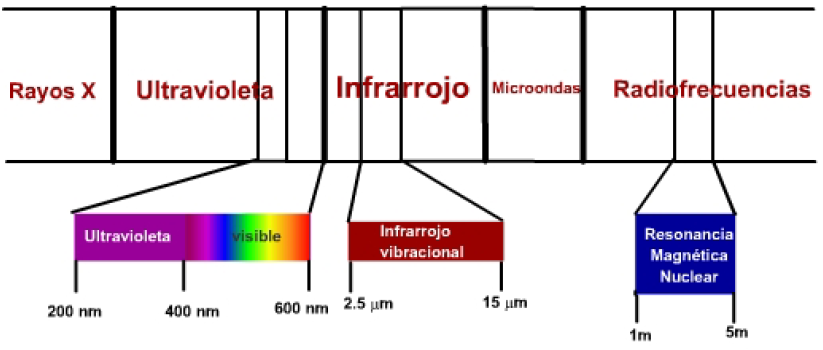
- Visible-ultraviolet radiation has adequate energy to produce molecular electron transitions at higher energy levels. It is the so-called UV spectroscopy, whose usefulness is mainly limited to the determination of molecules with unsaturations.
- Infrared radiation produces transitions between vibrational levels of a molecule. The bonds between the atoms of a molecule are not rigid, but vibrate around an equilibrium position, and infrared radiation is capable of taking these bonds to higher vibrational energy levels. This is called infrared (IR) spectroscopy.
- Radio waves have adequate energy to cause atomic nuclei, subjected to a magnetic field, to resonate. This technique is called nuclear magnetic resonance (NMR) spectroscopy.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 28205
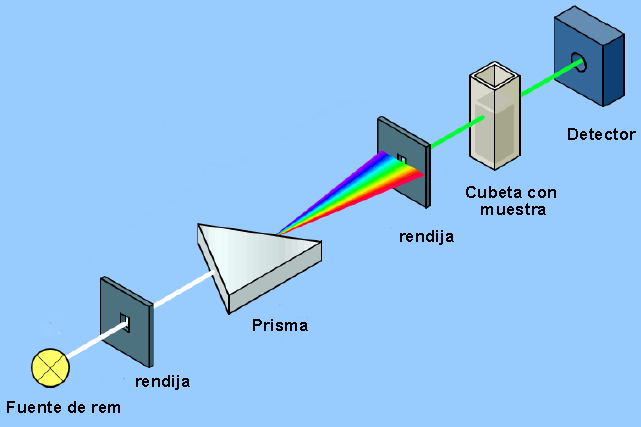
The instrument that allows detecting the interaction between electromagnetic radiation and matter is called a spectrophotometer and its basic structure can be seen in the following diagram.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 39520
Organic molecules distribute their electrons in different electronic levels called molecular orbitals. The lowest energy molecular orbitals are $\sigma$, followed by $\pi$ orbitals. When in the molecule there are atoms with free electronic pairs (oxygen, sulfur, nitrogen, halogens) we will have non-bonding levels n. These electronic levels are arranged in the bonding region of the diagram. In the antibonding region we find the molecular orbitals $\pi^{\ast}$ and $\sigma^{\ast}$. A molecule in its ground state has bonding and nonbonding orbitals occupied and antibonding orbitals unoccupied.
From what has been discussed above, it can be deduced that the electronic transitions must start from the bonding and nonbonding orbitals ending in the antibonding ones. Thus we can have the 5 types of electronic transitions shown in the following diagram.
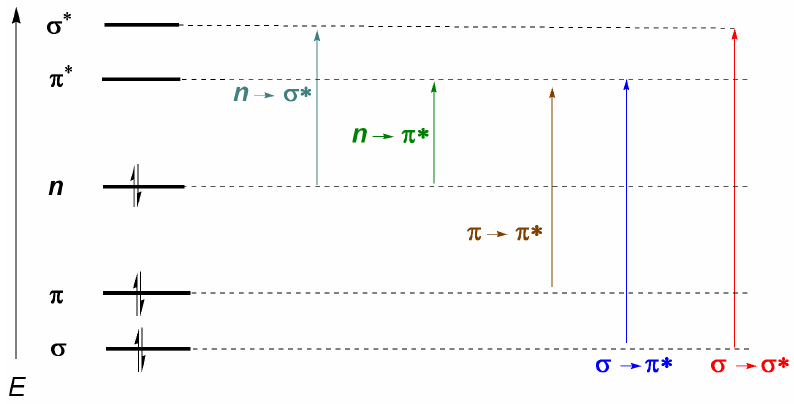
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 50042
The Lambert-Beer Law introduces the concept of absorbance (A) of a sample as $A=log\frac{I}{I_0}$. Where $I_0$ represents the intensity of the incident light and I the intensity of the light passing through the cell. We can also express absorbance as a function of cuvette length and solute concentration. \begin{equation} A=log\frac{I_0}{I}=\epsilon\cdot c\cdot l \end{equation} Where $l$ is the length of the cuvette in cm, $c$ represents the concentration of solute in mol/l and $\epsilon$ is the molar absorptivity (molar extinction coefficient) measured in l/mol.cm.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 24241
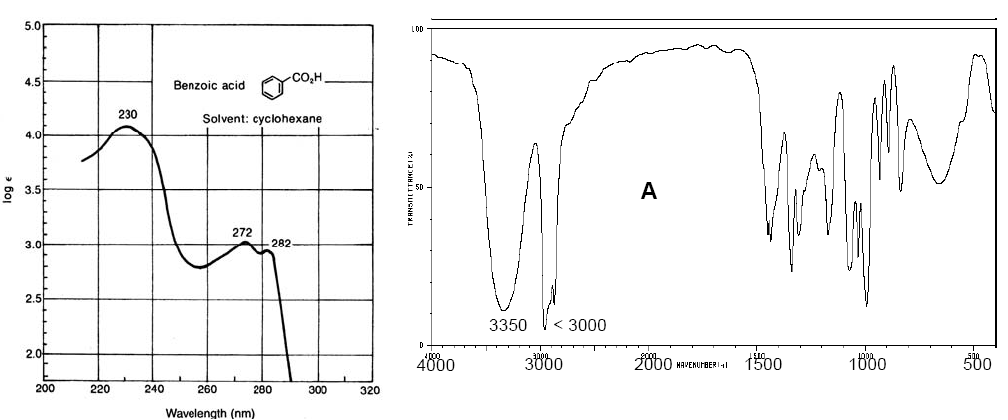 Vis-UV spectrum of benzoic acid (left). IR spectrum of cyclopentanol (right)
Vis-UV spectrum of benzoic acid (left). IR spectrum of cyclopentanol (right)
Vis-UV spectra have a lower resolution than IR because each electronic level is divided into vibrational levels and these in turn into rotational levels, such that an electronic transition consists of a large set of rotational-vibrational transitions. . IR spectra also have bands of considerable amplitude due to rotational transitions that occur simultaneously with vibrational transitions.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 63809
Chromophore groups are the functional groups of the molecule responsible for absorption. Mainly they are: carbon-carbon double and triple bonds, aromatic systems, carbonyl group, imino (C=N), diazo (N=N), nitro and CY bonds (Y is an atom with lone pairs).
Auxochromic groups are substituents on the chromophore and alter $\lambda_{max}$ and/or $\epsilon_{max}$. The methyl, halogen, hydroxy, alkoxy, and amino groups are auxochromes.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 34572
- Alkanes. Its absorption bands are due to $\sigma\rightarrow\sigma^{\ast}$ transitions of CC and CH bonds. These transitions are of high energy and take place at wavelengths below 150 nm, therefore not observable in conventional spectrophotometers. This characteristic allows them to be used as solvents for the sample to be analyzed, since they do not interfere with their signals.
- Alkenes and alkynes. They show absorption bands due to $\pi\rightarrow\pi^{\ast}$ transitions of the CC triple bond. This transition is of lower energy than in the case of alkanes and appears at longer wavelengths (alkenes: 175 nm; alkynes: 170 nm). The double and triple bond are the chromophore groups of these molecules.
- Ethers, thiols, sulfides, amines: In this case, the chromophore group is formed by the heteroatom (O,S,N) and the atoms that link it. The heteroatom presents lone pairs and the transition that produces the absorption is $n\rightarrow\sigma^{\ast}$. This absorption band appears around 175-200 nm for alcohols, ethers and amines, moving to 200-220 nm for sulfides.
- Aldehydes, ketones, acids and derivatives. The chromophore group of these compounds is carbonyl (C=O). Since oxygen has lone pairs, the transition with the lowest energy is $n\rightarrow\pi^{\ast}$, but it is a forbidden transition ($\epsilon_{max}=15$), since there is no overlap between the orbitals involved. The next lowest energy transition is $\pi\rightarrow\pi^{\ast}$, observable at $\lambda_{max}=188\;nm$, with a molar absorptivity of 900.
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 23532
Conjugated systems absorb at longer wavelengths than unconjugated ones. As the conjugation increases, the energy difference between HOMO and LUMO decreases and the radiation necessary to produce the transition $\pi \rightarrow \pi^{\ast}$ decreases its wavelength.
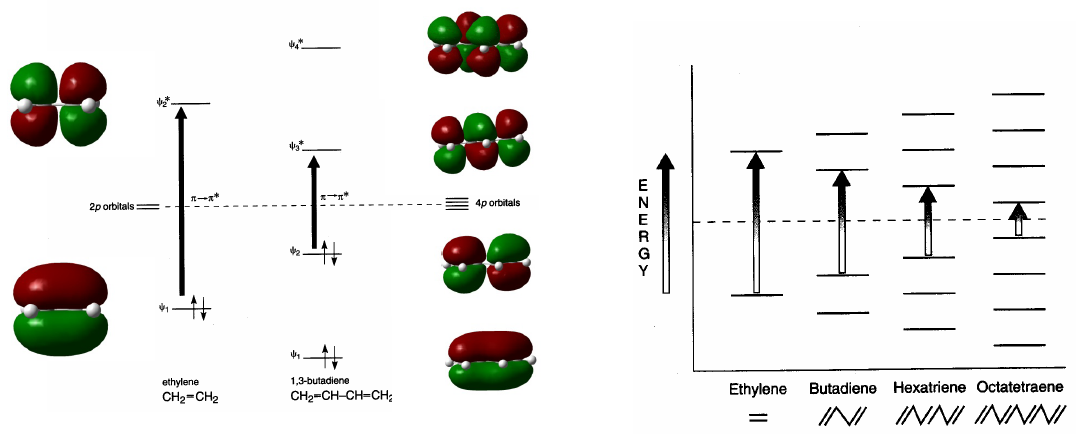
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 16299
The \alpha,\beta$-unsaturated molecular orbital diagram is constructed from the ethene and carbonyl molecular orbitals
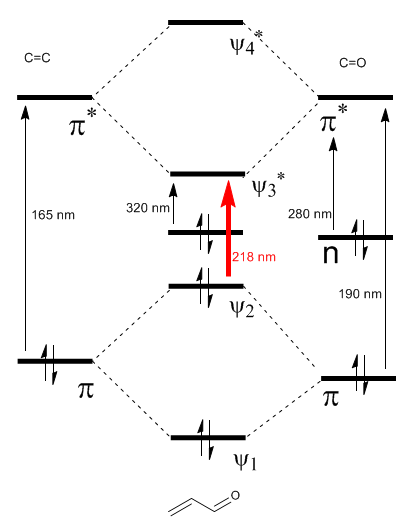
Read more: Bathochromic effect on $\alpha,\beta$-unsaturated carbonyls
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 15359
As can be seen in the diagram, the conjugation with the lone pairs of group X produces an approach between the HOMO and LUMO orbitals, giving rise to a transition of lower energy (longer wavelength) than in ethene.
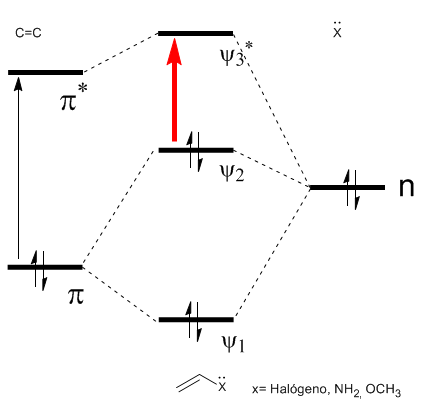
- Details
- Germán Fernández
- Visible-Ultraviolet Spectroscopy
- Hits: 28159
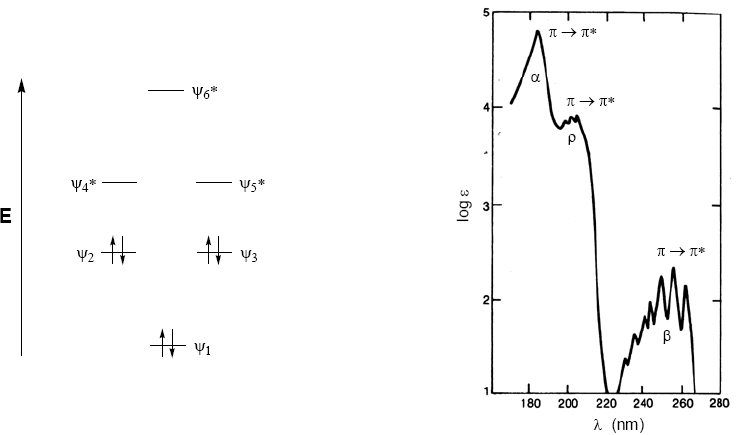
The absorption spectrum of benzene consists of three bands at 184, 204, and 256 nm, often called $\alpha$, p, and $\beta$. The $\alpha$, p bands are also known as primary and the $\beta$ secondary band. The secondary band is wide due to its vibrational structure.