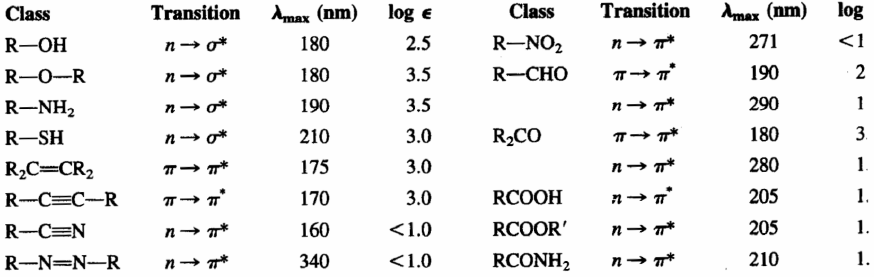
- Alkanes. Its absorption bands are due to $\sigma\rightarrow\sigma^{\ast}$ transitions of CC and CH bonds. These transitions are of high energy and take place at wavelengths below 150 nm, therefore not observable in conventional spectrophotometers. This characteristic allows them to be used as solvents for the sample to be analyzed, since they do not interfere with their signals.
- Alkenes and alkynes. They show absorption bands due to $\pi\rightarrow\pi^{\ast}$ transitions of the CC triple bond. This transition is of lower energy than in the case of alkanes and appears at longer wavelengths (alkenes: 175 nm; alkynes: 170 nm). The double and triple bond are the chromophore groups of these molecules.
- Ethers, thiols, sulfides, amines: In this case, the chromophore group is formed by the heteroatom (O,S,N) and the atoms that link it. The heteroatom presents lone pairs and the transition that produces the absorption is $n\rightarrow\sigma^{\ast}$. This absorption band appears around 175-200 nm for alcohols, ethers and amines, moving to 200-220 nm for sulfides.
- Aldehydes, ketones, acids and derivatives. The chromophore group of these compounds is carbonyl (C=O). Since oxygen has lone pairs, the transition with the lowest energy is $n\rightarrow\pi^{\ast}$, but it is a forbidden transition ($\epsilon_{max}=15$), since there is no overlap between the orbitals involved. The next lowest energy transition is $\pi\rightarrow\pi^{\ast}$, observable at $\lambda_{max}=188\;nm$, with a molar absorptivity of 900.
The following table shows the absorptions of the main chromophore groups.