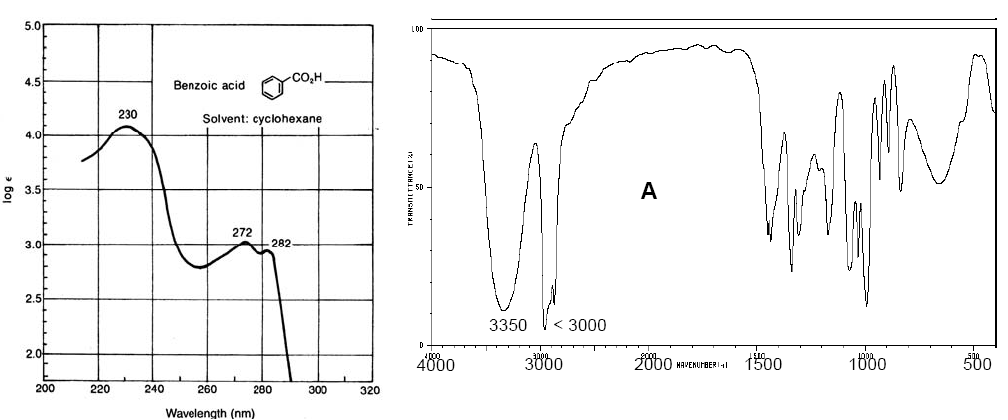
 Vis-UV spectrum of benzoic acid (left). IR spectrum of cyclopentanol (right)
Vis-UV spectrum of benzoic acid (left). IR spectrum of cyclopentanol (right)
Vis-UV spectra have a lower resolution than IR because each electronic level is divided into vibrational levels and these in turn into rotational levels, such that an electronic transition consists of a large set of rotational-vibrational transitions. . IR spectra also have bands of considerable amplitude due to rotational transitions that occur simultaneously with vibrational transitions.