There are three zones of the electromagnetic spectrum with special interest in the determination of chemical compounds:
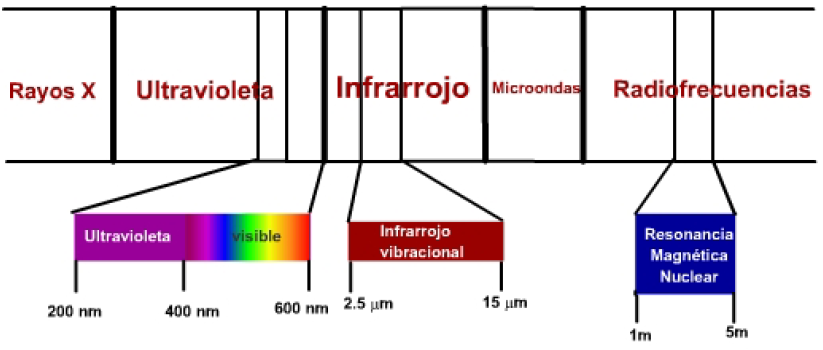
- Visible-ultraviolet radiation has adequate energy to produce molecular electron transitions at higher energy levels. It is the so-called UV spectroscopy, whose usefulness is mainly limited to the determination of molecules with unsaturations.
- Infrared radiation produces transitions between vibrational levels of a molecule. The bonds between the atoms of a molecule are not rigid, but vibrate around an equilibrium position, and infrared radiation is capable of taking these bonds to higher vibrational energy levels. This is called infrared (IR) spectroscopy.
- Radio waves have adequate energy to cause atomic nuclei, subjected to a magnetic field, to resonate. This technique is called nuclear magnetic resonance (NMR) spectroscopy.
Both the vibrational, electronic and nuclear spin energy levels are quantized and the energies necessary to promote the system from a lower level to a higher one are given by discrete values, characteristic of each system. A molecule will absorb electromagnetic radiation if the product $h\nu$ coincides with the energy difference between the lower level it is in and the higher one it promotes.