Synthesis of
Benzofurans and Benzothiophenes
(By the method of disconnections)
1. Synthesis of Benzofurans
Benzofuran, usually called coumarone, It is a colorless liquid, which is isolated from coal tar and is much more stable to chemical attack than furan.
The most classic syntheses for the preparation of benzofurans will be mentioned and developed:
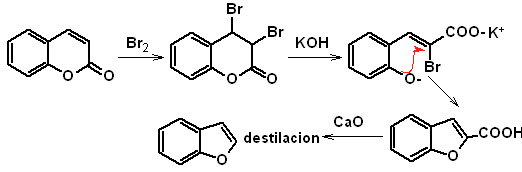
to. From the coumarin
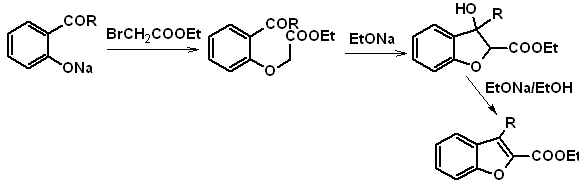
b. From an internal Claisen condensation reaction
c.
Starting of a Claisen rearrangement
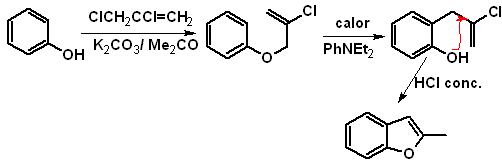
Propose a synthesis design, for the following benzofurans:
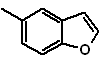
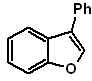
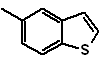
: | MOb 127
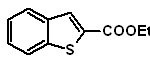
| … | MOb 128
|
MOb 127, Retrosynthetic analysis. The disengagement strategy, in
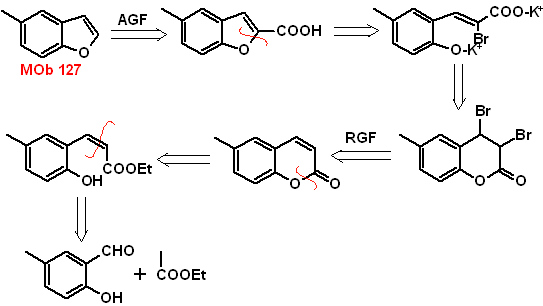
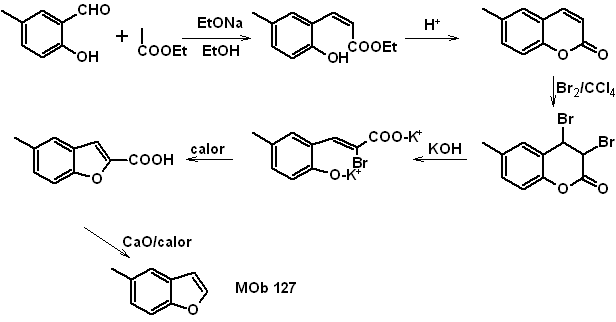
Synthesis. The intermediate 2-hydroxy-5-methylbenzaldehyde is prepared from benzene. The coumarin derivative that is formed is halogenated, hydrolyzed in a KOH sol and subsequently heated with CaO, to decarboxylate and thus form
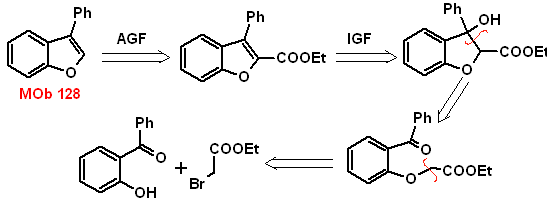
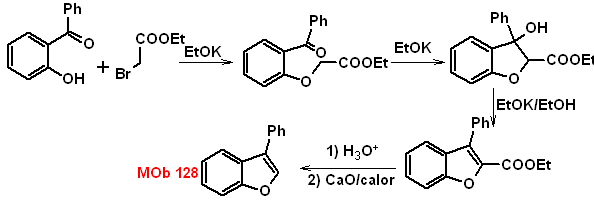
MOb 128. Retrosynthetic analysis.
Benzothiophene is the most important sulfur-containing impurity of technical naphthalene, so benzothiophene itself has little commercial value, but some of its derivatives in the form of indigo dye have great value.
The synthetic methods of benzothiophene are similar to those used for benzofuran, but the following methods can be mentioned.
to.
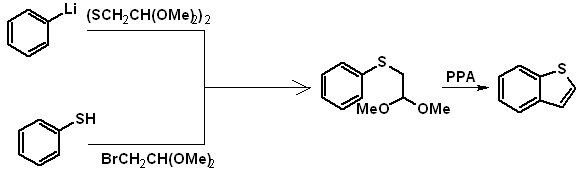
From suitable benzothioderivatives:
b.
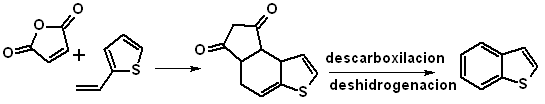
Through a Diels-Alder reaction
c.
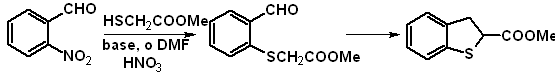
By displacement of ortho groups to a cyano group (leading to 3-amino compounds) or aldehyde on a benzene ring by a suitable sulfur nucleophile and subsequent cyclization.
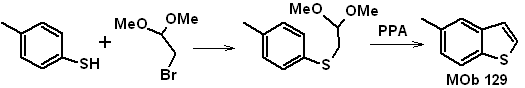
Propose a feasible synthesis plan for the following molecules:
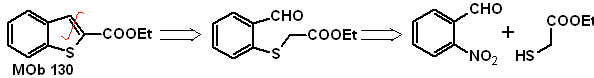
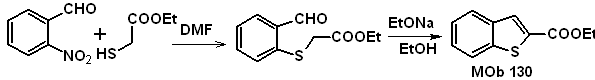
MOb 129 | ….. | MOb 130
|
MOb 129 . Retrosynthetic analysis.
Synthesis