ORGANIC SYNTHESIS
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 26054
SYNTHESIS OF HETEROCYCLES BY INTRAMOLECULAR CYCLATION
The construction of heterocyclic systems also uses these same reactions, with the particularity that the heterocyclic system must be present or contain at least one atom other than carbon. The most common are nitrogen, oxygen, sulfur and phosphorus.
The cyclic system of the molecule to be synthesized can come from the modification of a cyclic system present in one of the reagents involved in the synthesis or be the result of cyclization of non-cyclic antecedents and that has been built in the development of the synthesis by intramolecular cyclization or by methods based on intermolecular cyclizations (cycloadditions).
1. Intramolecular cyclization
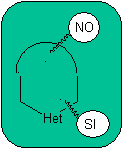
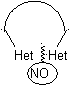
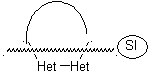
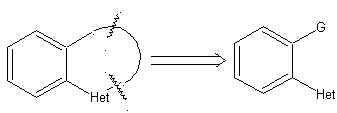
The general rules for the disconnection of heterocycles originating from an intramolecular cyclization, were adequately systematized by JI Borrell , the same as those assumed in this section (Het = N, O, S)
1.
In the synthesis of a monocyclic compound, ring closure generally involves the formation of a carbon-heteroatom bond.
Model: |
|
Example: |
|
|
|
Example : |
|
Model: |
|
Example: |
|
Read more: Synthesis of heterocycles by intramolecular cyclization
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 22339
MOb: 75 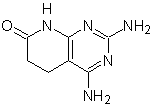 | MOb: 76 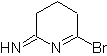 |
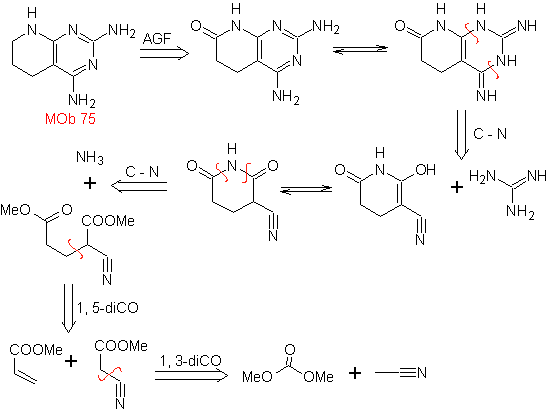
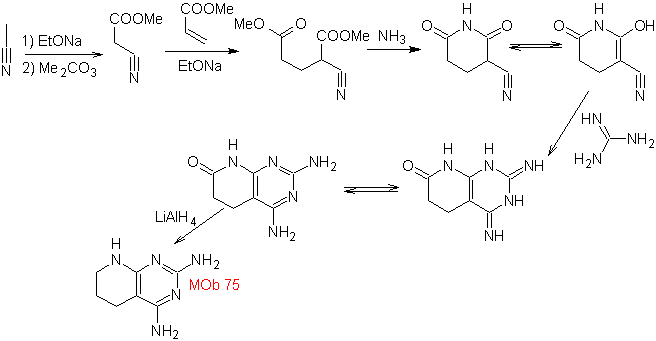
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 26907
Synthesis of heterocycles with several heteroatoms
Heterocyclic compounds, as already mentioned, have a wide range of applications: they predominate among the compounds used as pharmaceuticals, agrochemicals, and for veterinary use; they are used as polishing additives, antioxidants, corrosion inhibitors, as dyes and pigments; and in many more applications.
Therefore, it is reasonable that currently much of the research in chemistry deals with the synthesis and properties of heterocyclic compounds. To this end, this article is oriented, which aims to provide chemistry students with basic retrosynthesis tools.
The disconnection process for molecules with several heteroatoms can be carried out for each carbon-heteroatom bond, according to the previously studied models or simultaneously, for which affordable polyheteroatom reagents are used.
1. Distance heteroatoms (1, 2)
The most representative and usual reagents are hydrazines and substituted hydrazines, as well as hydroxylamines.
hydrazine |
hydroxylamine |
Propose a synthesis design, from simple materials, for the following molecules:
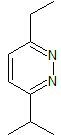
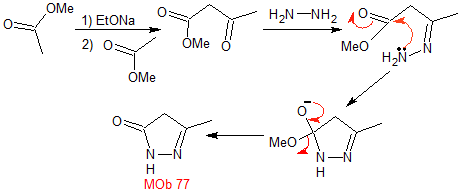
mob 77
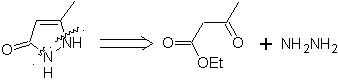
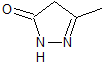
pyrazoles | MOb 78
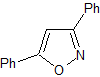
isoxazoles | MOb 79
pyridazines |
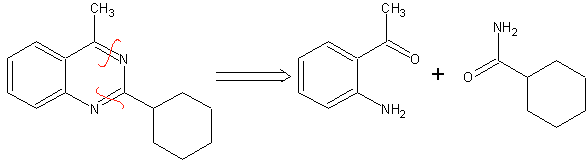
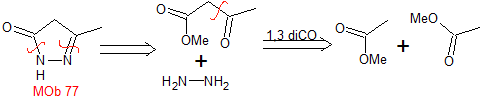
MOb 77 . Retrosynthetic analysis. MOb is a pyrazole derivative and is directly disconnected by CN bonds, to generate simple precursors such as hydrazine and a 1,3-diCO compound.
|
synthesis . Methyl acetate is a good precursor to form the compound 1,3-diCO, which combines with hydrazine to generate MOb 77. |
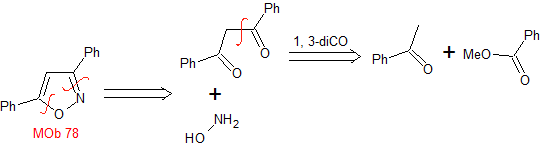
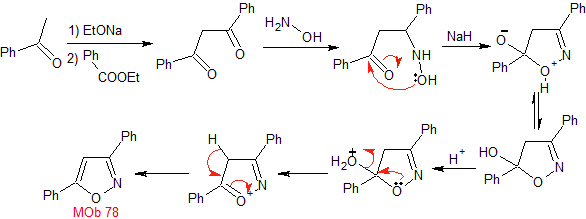
MOb 78- Retrosynthetic analysis .
|
Synthesis. Bezophenone and ethyl benzoate make it possible to form the required 1,3-diCO, to react in a slightly acidic medium with hydroxylamine and after adding NaH, cyclization occurs, which requires more acid to
dehydrate and finally produce
|
Read more: Synthesis of heterocycles with several heteroatoms
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 23823
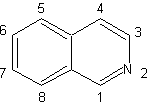
Isoquinoline synthesis
(By the method of disconnections)
| Isoquinolines differ structurally from quinolines in the position nitrogen, since the latter is not fused, so it presents an "aliphatic reactivity". It is not found free in nature, but the isoquinoline cycle is found in some alkaloids, in aromatic or reduced form, eg papaverine. |
The best known synthetic methods for the preparation of isoquinolines start with 2-phenylethylamines and involve a cyclization through an additional carbon provided by the carbonyl group of another compound.
The main synthetic methods are: the Pomeranz-Fritsch synthesis, the Bischler-Napieralski synthesis, the Pictet-Gams synthesis and the Pictet-Spengler synthesis.
1. Synthesis of POMERANZ-FRITSCH.
This method of synthesis of isoquinoline occurs in two stages:
to.
First, benzaldehyde (1,3-electrophile-nucleophile) is condensed with aminoacetaldehyde diethylacetal (1,3-nucleophile-electrophile) to form a stable aldimine.
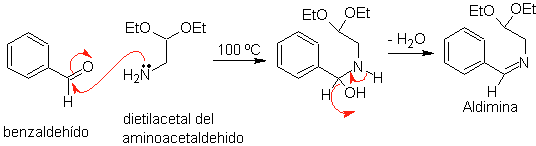
b.
Subsequently, the aldimine cyclizes in a strong acid medium, to an imine, with simultaneous elimination of ethanol, to produce an isoquinoline.
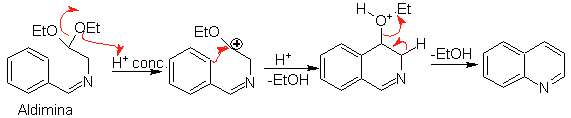
This second stage, being an electrophilic substitution, is subject to the effect that the electron-donating or accepting substituents have on the benzene ring in said reaction. However, due to the hydrolysis of the imine formed, in the strong acid medium used in the reaction, the yield of the process is reduced.
This method allows access to C-1 substituted isoquinolines, for which aromatic ketones have been tested, with very low yields. However, there has been greater success using the variant of appropriately substituted benzylamines as 1,4-dinucleophiles and glyoxal diethylacetal as 1,2-dielectrophiles.
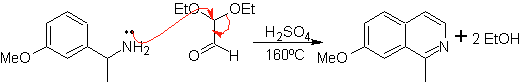
Something that must be made clear is that the Pomeranz-Fritsch method and its variant, previously analyzed, do not allow the preparation of isoquinolines substituted at C-3 and C-4 of the heteroatom. The retrosynthetic analysis of this method shows the possible intermediates involved in the reaction and the probable starting materials.
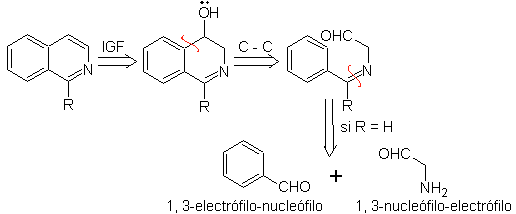
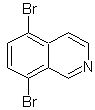
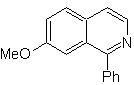
Propose a synthesis design for each of the following isoquinolines: | MOb 107
| MOb 108
|
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 17777
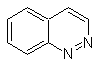
Synthesis of BENZODIAZINES
(By the method of disconnections)
The structures of benzodiazines are found in many alkaloids, mainly as a quinazolone ring system. The other derivatives of benzodiazine, such as cinnolines, quinoxalines and phthalizines, are also an important part of many drugs with a spectrum of significant use, which makes them, in general, very important in organic synthesis and particularly in pharmacochemistry. Thus, they can be found as anti-inflammatory, antihypertensive, antibacterial, analgesic, antibiotic, etc.
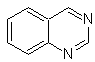
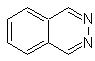
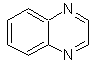
cinnoline |
quinazoline | |
phthalizine |
Quinoxaline |
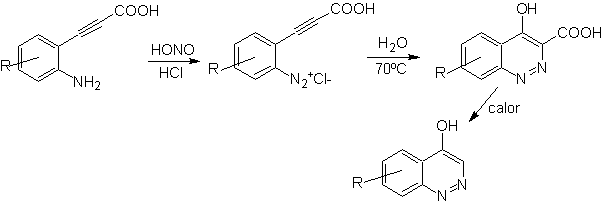
Synthesis of the Cinnolines
According to the structure that cinnoline presents, there are the following options for its synthesis:
![]() von Richter synthesis:
von Richter synthesis:
![]() Widman–Stoermer synthesis:
Widman–Stoermer synthesis:
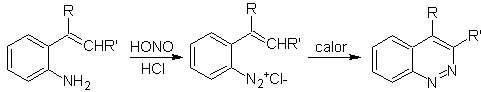
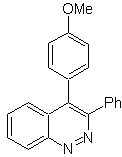
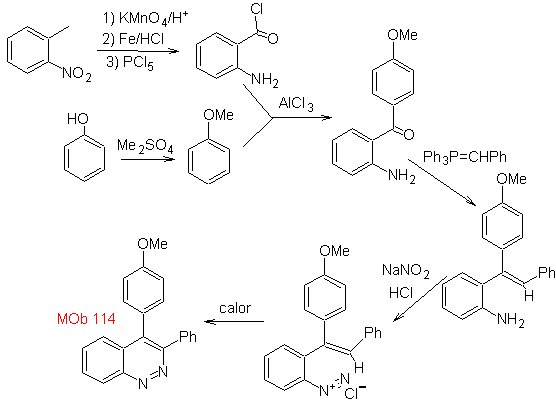
Propose a synthesis plan for the following molecule : | MOb 114
|
MOb 114 . Retrosynthetic analysis.
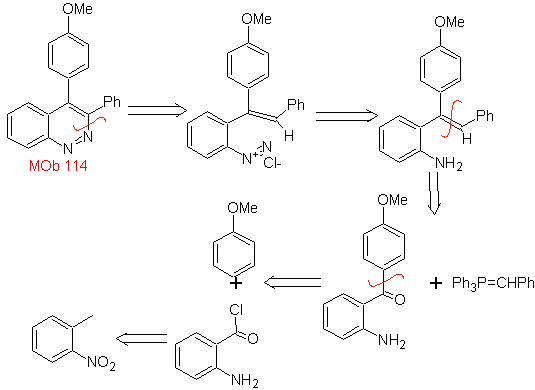
Synthesis The formation of
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 65095
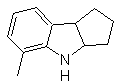
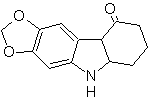
Synthesis of INDOLES
(By the method of disconnections)
The indole ring system has been found in many natural compounds of great chemical and biochemical interest, which is why it is said to be the most abundant in nature. Thus, tryptophan is an essential amino acid, indigo is a dye, and indolyl-3-acetic acid is a plant growth hormone. On the other hand, interest in these molecules arises from their pharmacological use, examples being sumatriptan (antimigraine) and frovatriptan, also an antimigraine.
Indole is a colorless crystalline solid with a mp 52°C, easily soluble in most organic solvents and crystallizes from water, has a pleasant odor and is therefore also used as a perfume base.
It was first prepared in 1866 by heating oxindole with zinc dust and has become an important commercial product. Baeyer in 1869 proposed the following synthesis:
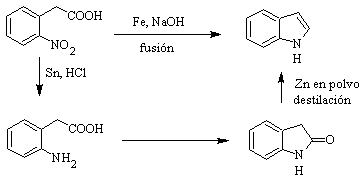
The classical synthesis methods for indoles are those of Fischer, Bischler, Reissert and Leimgruber-Batcho, Bartoli, Larock, Gassman, Sugasawa, Fukuyama, Hegedus, and Dobbs.
1.
FISCHER synthesis
It consists of heating phenylhydrazones of ketones or aldehydes, with anhydrous zinc chloride, boron trifluoride, polyphosphoric acid, or some other acid catalyst, to produce indoles. An acid-catalyzed rearrangement of a phenylhydrazone occurs with elimination of water and NH 3 . Electrodonor groups favor cyclization and electroattractors hinder it.
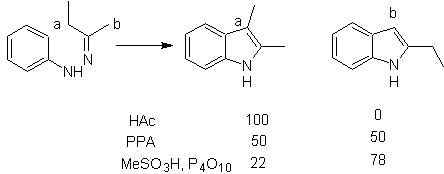
With asymmetric ketones, the intramolecular cyclization of the hydrazone can lead to two isomeric indoles in different proportions depending on the conditions used; in strongly acidic media, the less substituted indole can predominate.
When there are meta substituents, with respect to the hydrazone nitrogen, cyclization can take place in two positions, leading to two isomeric indoles:
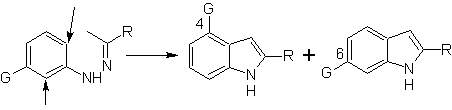
If the substituent G is electro-withdrawing, the two isomers (4- and 6-) are formed in approximately the same proportion. On the other hand, if G is an electron-donating substituent, the 6-substituted isomer is formed mainly. The retrosynthetic analysis of the indole formed by the Fischer synthesis can be considered as follows:
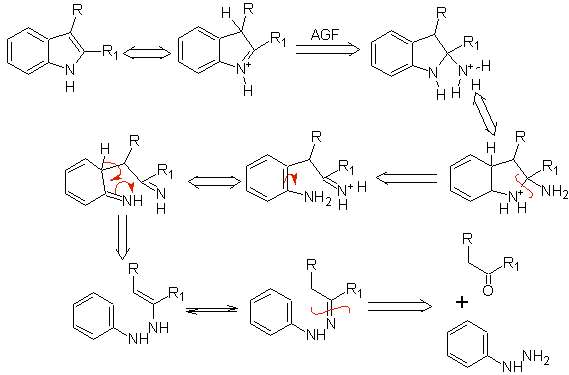
Propose a synthesis plan for the following molecules: | MOb 119
| MOb 120
|
MOb 119. Retrosynthetic analysis. The fundamental disconnection in the indoles that are supposed to be formed by the Fischer synthesis corresponds to a retro-transposition, which is shown in the disconnection of
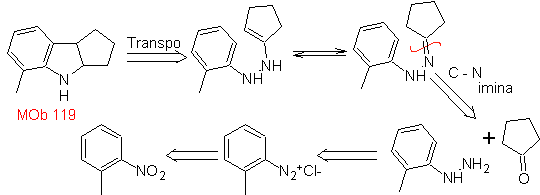
Synthesis : From ortho-nitrotoluene, the intermediate derivative of phenylhydrazine is generated, necessary in the synthesis of Fischer indoles, the imine is formed with a cyclopentanone, and by heating
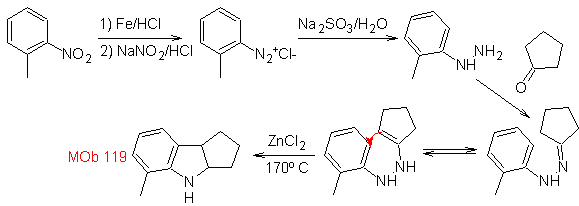
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 22542
Synthesis of
Benzofurans and Benzothiophenes
(By the method of disconnections)
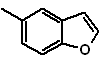
1. Synthesis of Benzofurans
Benzofuran, usually called coumarone, It is a colorless liquid, which is isolated from coal tar and is much more stable to chemical attack than furan.
The most classic syntheses for the preparation of benzofurans will be mentioned and developed:
to. From the coumarin
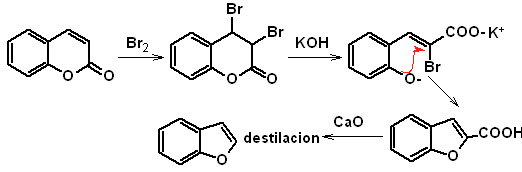
b. From an internal Claisen condensation reaction
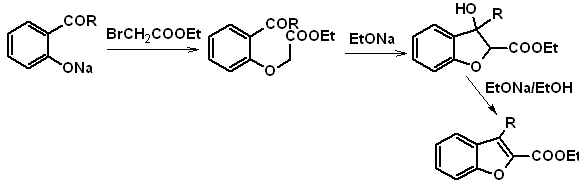
c.
Starting of a Claisen rearrangement
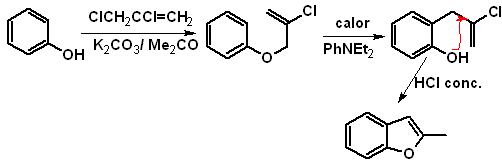
Propose a synthesis design, for the following benzofurans:
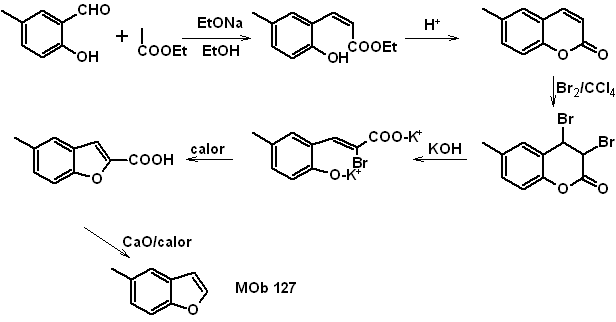
: | MOb 127
| … | MOb 128
|
MOb 127, Retrosynthetic analysis. The disengagement strategy, in
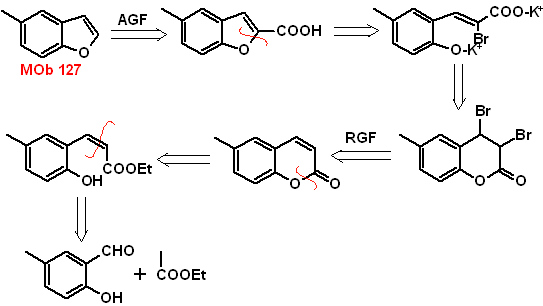
Synthesis. The intermediate 2-hydroxy-5-methylbenzaldehyde is prepared from benzene. The coumarin derivative that is formed is halogenated, hydrolyzed in a KOH sol and subsequently heated with CaO, to decarboxylate and thus form
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 48928
SULFAMIDE SYNTHESIS
It is known that sulfonamides were the first antimicrobials to be used systemically. Its chemical structure is a benzene nucleus with amino groups that give it its activity. The amino group is acetylated in the liver, inactivating it. Depending on the substituent in said sector the drug is more active.
Given its similarity to para-aminobenzoic acid, it behaves as a competitive inhibitor of this substance, which is necessary together with dihydropteridine to synthesize dihydrofolic acid, an intermediate compound in the folate synthesis pathway.
Unlike more advanced organisms, bacteria need to synthesize their own folates [they do not acquire it from the environment], so sulfonamides, by inhibiting this process, inhibit the processes of synthesis of nucleic acids and are BACTERIOSTATIC.
TRIMETROPRIM
Trimethoprim is a derivative of 2,4 diaminopyrimidines such as | This compound INHIBITS the enzyme dihydrofolate reductase and prevents the formation of tetrahydrofolic acid, that is, they act in the same metabolic pathway as sulfonamides, but in a subsequent enzymatic reaction. Trimethoprim is never used alone, but when associated with sulfonamides they are potentiated in such a way that they become BACTERICIDAL, decrease the possibility of generating resistance and increase the antimicrobial spectrum. The association between sulfamethoxazole and trimethoprim is fixed: 1:5. For example, the commercial preparations Cotrimoxazole [forte or not] come with this reason. |
Sulfonamides, they are generally classified according to the duration of their action and the way the drug is applied, as well as other characteristics. Depending on the mode of action, sulfonamides can be:
to)
Short or intermediate acting sulfonamides.
to. General use sulfonamides
Yo. Sulfathiazole
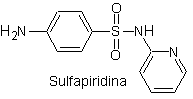
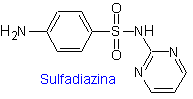
ii. sulfadiazine
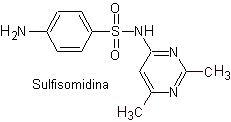
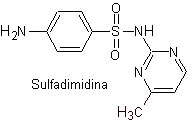
iii. Sulfadimidine
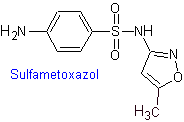
iv. Sulfamethoxazole (alone or associated with trimethoprim: cotrimoxazole)
b. Highly soluble compounds initially used in the treatment of urinary tract infections.
Yo. Sulfisoxazole
ii. sulfamethizole
iii. Sulfasomidine
b)
Long-acting sulfonamides.
iv. sulfamethoxypyridazine
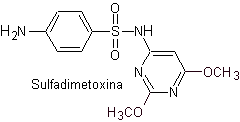
v. sulfadimethoxine
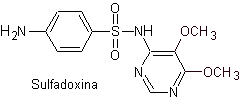
saw. Sulfadoxine
c)
Sulfonamides limited to the gastrointestinal tract
vii. Sulfaguanidine
viii. Sulfatalidine
ix. Sulfasuxidine
x. Sulfazolazine
d) Topical Sulfonamides.
xi. mafenide acetate
xii. silver sulfadiazine
xiii. sul facetamide sodium
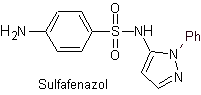
Main sulfas:
| ……… |
|
|
|
| …….. |
|
|
|
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 26756
Synthesis of Fluoroquinolone Antibiotics
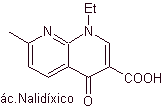
Quinolones belong to a group of synthetic antibacterial agents. The oldest agent in this family, nalidixic acid, used in the early 1960s, has a good spectrum against enterobacteria (limited antibacterial spectrum) but its pharmacokinetics are not very favorable for routine clinical use due to its low bioavailability. in tissues and its short half-life.
For this reason, it was necessary to synthesize new antibacterials of this family to improve the spectrum of activity, the pharmacokinetic profile, reduce adverse effects and the appearance of bacterial resistance. This new group are the so-called fluoroquinolones, generated during the 80s.
|
Many researchers agree that the Gould-Jacobs reaction , is the main basis for the synthesis of the first quinolones for pharmacological use, which occurred in the 1960s, this reaction presents the following sequence:
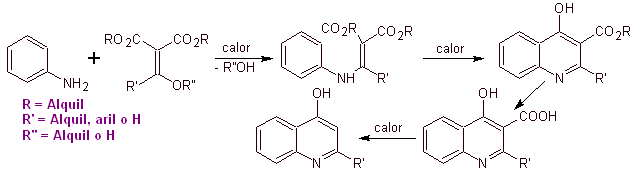
In the following years, fluorine in position 6 and various groups of heterocycles in position 7 have been introduced into the basic ring of benzoquinolones, giving rise to fluoroquinolones with a greater antibacterial spectrum.
Important parts of the methodologies used in these syntheses have been compiled by Leyva S and Leyva E in very good work from a biochemical perspective.
The disconnection method applied to the report of the syntheses under study and the reactions of the first stages of the syntheses are the sole responsibility of the author of this monograph. .
i) Synthesis reported by Koga H. et al.
Retrosynthetic analysis:
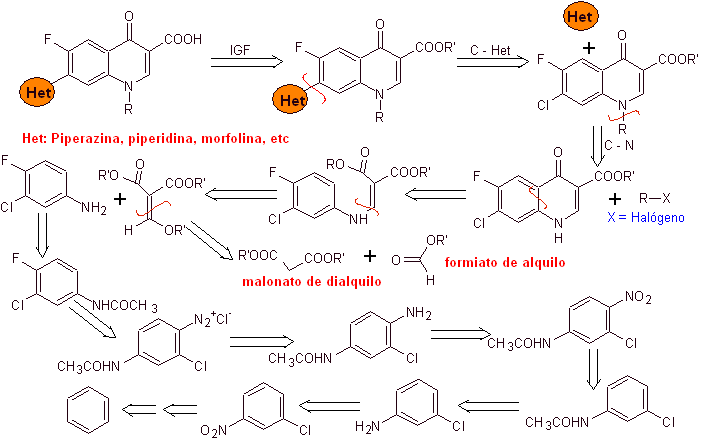
Synthesis: It starts from benzene to form 3-chloro-4-fluoroaniline, which reacts with diethyl EMME to produce the corresponding acrylate, which upon heating forms a cyclic compound. This compound in turn is reacted with an alkylating agent and subsequently the nitrogenous heterocyclic compound is introduced, to finally hydrolyze and obtain the target molecule.
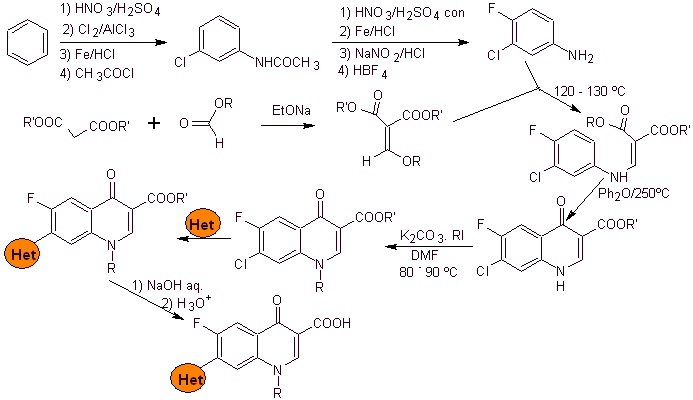
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 38053
Synthesis of local anesthetics derived from benzoic acid
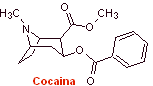
The properties of the alkaloids isolated from the leaves of the coca plant were discovered for the first time by Gaediche in 1855, the purification and isolation of the active principle called cocaine by Albert Nieman in 1860, practically began the history of local anesthetics. . Subsequently, Einhorn introduced procaine (novocaine) as a local anesthetic in medicine in 1904. |
|
Since then, humanity has witnessed a continuous and sustained development of the synthesis of new molecules with anesthetic active principles:
![]() In 1925 Niescher synthesized nupercaine.
In 1925 Niescher synthesized nupercaine.
![]() In 1928 Von Eisleb tetracaine (pantocaine) and
In 1928 Von Eisleb tetracaine (pantocaine) and
![]() In 1946 Lofgren and Lundquist synthesized lognicaine (xylocaine or lidocaine).
In 1946 Lofgren and Lundquist synthesized lognicaine (xylocaine or lidocaine).
![]() Then in 1954 Af Ekenstam and Egner obtained the synthesis of mepivacaine (scandicaine).
Then in 1954 Af Ekenstam and Egner obtained the synthesis of mepivacaine (scandicaine).
![]() Later in 1960 and 1964 they were introduced in
Later in 1960 and 1964 they were introduced in
![]() Finally, in the following years, new anesthetics have been incorporated into medicine.
Finally, in the following years, new anesthetics have been incorporated into medicine.
Local anesthetics are drugs that, when applied to a specific area of the body, produce a temporary and reversible loss of sensitivity (thermal, painful and tactile), without inhibition of the patient's consciousness. The duration of the effect of the drug depends on the dose used, its chemical structure, the formulation and the pharmaceutical form of the drug.
In general, local anesthetic drugs respond to different chemical structures, but all of them have similar effects or different intensities of the anesthetic effect. However, an attempt can be made to group them into benzoic acid esters, aminobenzoic acid esters, Amides, etc.
1 . Chemical structure of local anesthetics
Local anesthetics are predominantly weak bases and are formed by an arene group, ester or amide, which gives the molecule lipophilic properties (which mainly determine the potency of the drug), an aliphatic tertiary amino group (alkyl or alicyclic), which gives the molecule its hydrophilic character, and an alkyl intermediate chain that joins the parts of the arene with the amine and is responsible for the level of toxicity of the drug.
Thus, the main local anesthetics used in the different medical disciplines can be found in the following groups:
to)
Amino esters of benzoic acid :
b)
Esters of m-aminobenzoic acid :
c)
Esters of p-aminobenzoic acid :
d) Amides:
and)
Ketones :
F)
other groups
2 . Synthesis of local anesthetics derived from amino esters of benzoic acid
The most representative drugs of this group are cocaine, hexylcaine, piperocaine, ethyl aminobenzoate, meprilcaine, amylocaine, cyclomethicaine and propanocaine. These names respond to
Read more: Synthesis of Local Anesthetics derived from benzoic acid
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 39727
Synthesis of local anesthetics derived from aminobenzoic acid
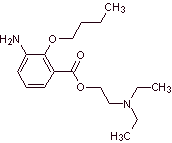
Local anesthetics derived from m-aminobenzoic acid
The most representative drugs of this group are
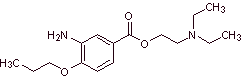
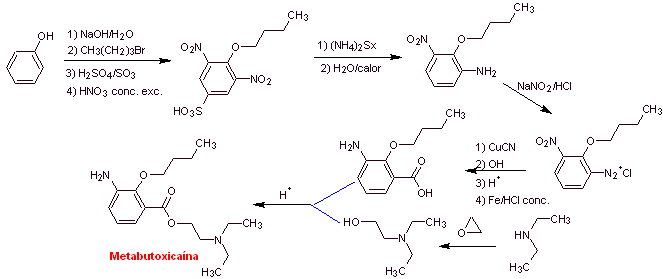
MOb 07: Metabutoxycain , marketed under the name primacaine , is another local anesthetic used in dentistry. Propose a synthesis design for this drug, starting from simple and affordable materials. |
|
Retrosynthetic analysis : Acyl-oxygen cleavage generates an amino alcohol cleavable to secondary amine and epoxide; the other precursor invites to prepare its carboxyl group by hydrolysis of a nitrile group, which will be placed on the benzene ring by the Sandmeyer reaction. The selective reduction of only one of the nitro groups is carried out with ammonium polysulfide or also with Na 2 S.
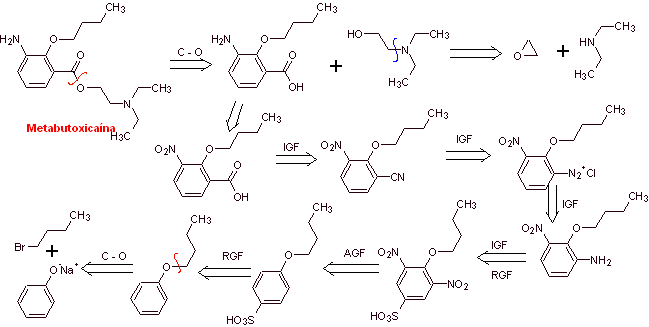
Synthesis of
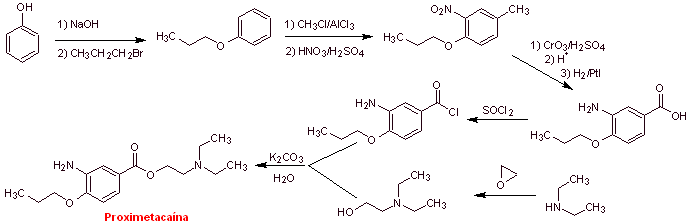
MOb 08: Proximethacaine (INN) or proparacaine , known by the trade names Alcaine, Ak-Taine, and others, is a topical anesthetic drug from the amino ester group. It is indicated for use as an ophthalmic anesthetic to reduce pain and discomfort in the eye. Propose a synthesis design for this anesthetic, starting from simple and affordable materials. |
|
Retrosynthetic analysis : The initial acyl-oxygen disconnection of proxymethacaine, again leads to an m-aminobenzoic acid, with an alkoxide substituent in the para position and an aminoalcohol that is formed from the corresponding amine and epoxide.
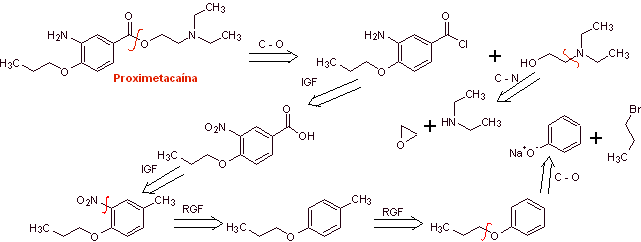
Synthesis of proximethacaine :
Read more: Synthesis of Local Anesthetics derived from aminobenzoic acid
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 31537
Synthesis of local anesthetics derived from phenylacetamides
A list of the main components of this group of anesthetics is presented next:
Lidocaine (lignocaine, xylocaine), mepivacaine, etidocaine, articaine (carticaine), bupivacaine, prilocaine, dibucaine (cincocaine), ropivacaine, trimecaine, butanylicaine, clibucaine, tolicaine, trimecaine, vadocaine, or xitazaine, anidicaine, dimethisoquin, oxetazin, pyrrocaine, paramoxin, properacaine, oxetacaine.
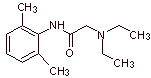
MOb 19 ; |
|
Retrosynthetic analysis :
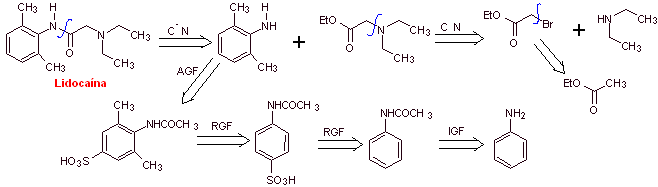
Synthesis of
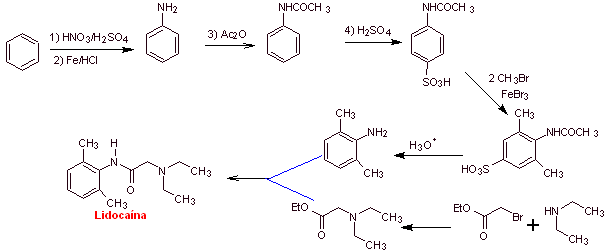
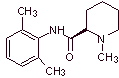
MOb 20: Mepivacaine is a local anesthetic of the amide type, it is faster acting than procaine, but its anesthetic effect is shorter than that of procaine. Like most anesthetics it is supplied as the racemate hydrochloride salt. |
|
Retrosynthetic analysis : The initial disconnection by the amide bond generates two precursor molecules, one of which, 2,6-dimethylaniline, has already been synthesized in
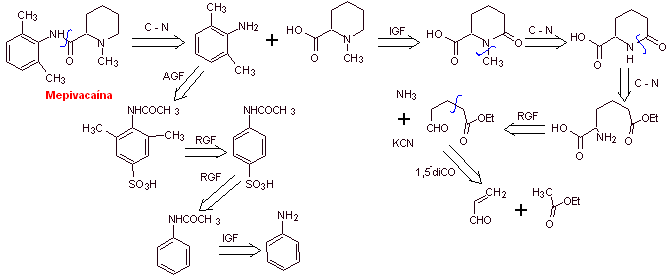
Synthesis of mepivacaine :
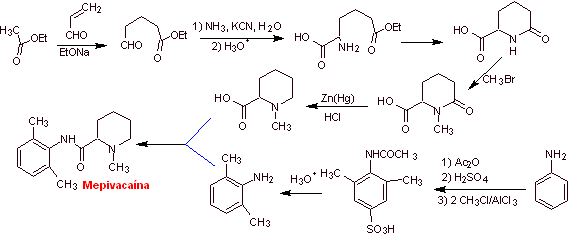
Read more: Synthesis of Local Anesthetics derived from phenylacetamides
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 19694
Synthesis of local anesthetics derived from various functional groups
There are analgesics, which, being derived from functional groups other than benzoic acid, aminobenzoic acid or phenylacetamide, also have analgesic properties similar to previously synthesized drugs. The most representative in this group are the following:
Phenacaine, promocaine (pramoxine), bucricaine, ethyl chloride, dimethisoquine, diperodon , ketocaine, myrtecaine, octacaine, dyclonine (diclocaine)
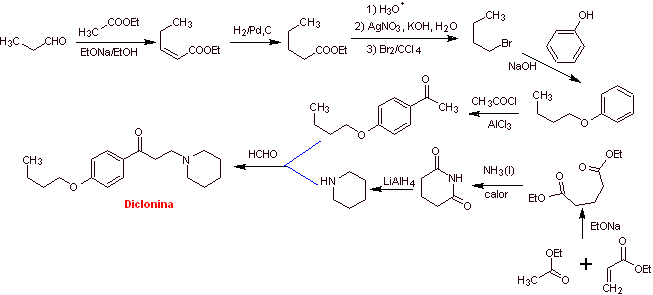
MOb 29 : Dyclonine is an oral anesthetic in Sucrets, one more of the throat lozenges. It is also found in some varieties of Cepacol sore throat spray. Propose the synthesis of the drug. | 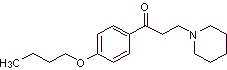 |
Retrosynthetic analysis : The structure of ketoamine allows us to propose a disconnection linked to the Mannich reaction (methyl ketone + formaldehyde + secondary amine). Subsequent disconnections of the precursor molecules are related to relatively simple, high-yield chemical reactions.
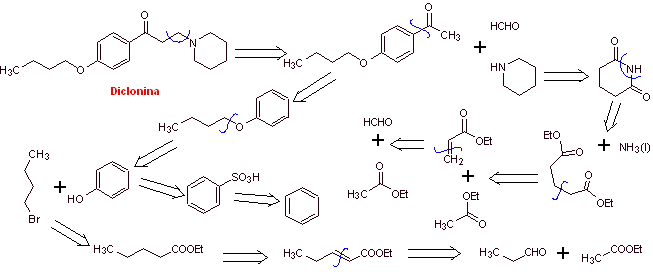
Synthesis of dyclonine :
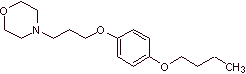
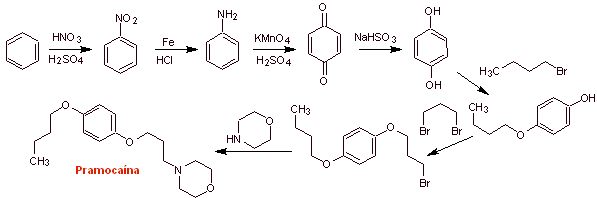
MOb 30 : Pramocaine ( also known as pramoxine), is a topical anesthetic used as an antipruritic. Its hydrochloride is soluble in water and therefore more easily absorbed by the skin. Propose a synthesis design for this molecule from simple and affordable materials. |
|
Retrosynthetic analysis :
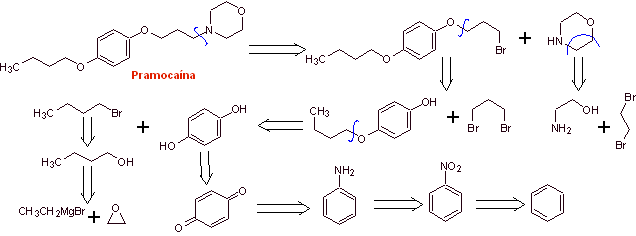
Pramocaine synthesis :
Read more: Synthesis of Local Anesthetics derived from various functional groups
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 14107
Polyaspartate synthesis (rootGrow)
1. Polyaspartate synthesis (rootGrow)
It is claimed that the rootGROW formula is related to that of nutra-sweet (aspartame), a powerful sweetener, which is being withdrawn or has already been withdrawn from the market, due to its harmful effects on people's health.
Nutra-sweet can be synthesized from phenylalanine, according to the following scheme:
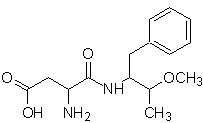
nutra sweet
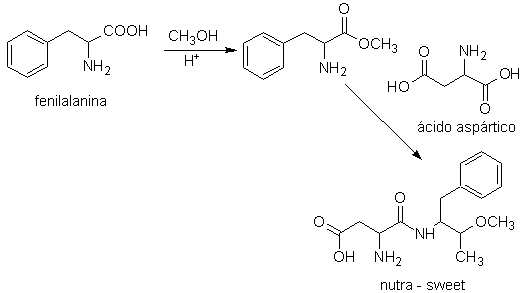
In turn, the aspartic acid, the fundamental base, to obtain the rootGrow, which can be dextrorotatory (D) or levorotatory (L), is prepared according to the following synthesis schemes:
Scheme A: (transamination process)
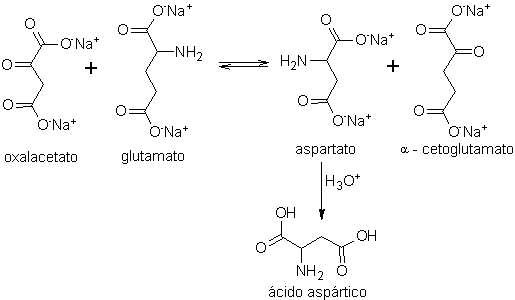
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 30087
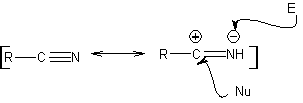
Synthesis of 1,3 and 1,5-dicarbonyl compounds
Propose a synthesis design by the disconnection method (synthon method) from simple and affordable materials, for the following molecules:
(Remember that if it is not possible to propose a direct disconnection, it will be necessary to resort to the strategy of previously functionalizing the MOb, until reaching an applicable disconnection model)
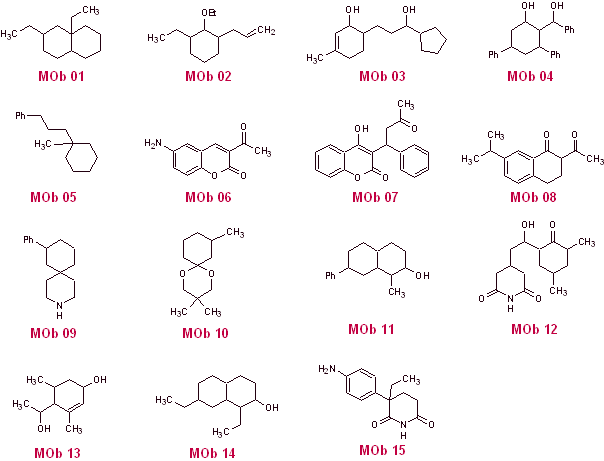
SOLUTIONS TO PROPOSED PROBLEMS
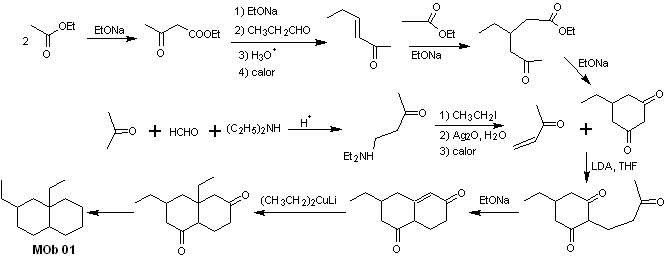
MOb 01 does not have any dioxygen ratio, an aspect that in some sense is an advantage for the chemist. That is, the strategy to be used will allow the search for dioxygenated relationships in the precursor molecule (synthetic equivalent) with a certain degree of freedom, that is, it can be postulated, depending on the structure of the MOb, a range of dicarbonyl and/or hydroxycarbonyl relationships, in positions relative 1.2, 1.3 , 1.4, 1.5 and/or 1.6 or their variants, such as α, β-unsaturated carbonyl compounds.
On this occasion, the synthesis will be exercised, resorting to the 1.3 and/or 1.5 dioxygenated ratios. In this way, the target molecule and precursors will be converted into disconnectable structures according to a known and pre-established pattern.
Solution MOb 01
Retrosynthetic analysis : In the molecule in question, one can start with perform an AGF, placing a C=O group in the structure of the precursor molecule, in such a position, which allows later to make another AGF, with a double bond located on the alpha and beta carbon with respect to the carbonyl, to proceed to disconnect it.
The α, β unsaturation, with respect to C=O, must be sought as the most substituted alkene of the alternatives that could exist. The presence of a substituent in the beta position at C=O leads us to think that it could have added as a nucleophile to an α, β unsaturated carbonyl compound, according to the Michael conjugate addition reaction. Based on these considerations, the following retrosynthetic analysis can be postulated for MOb 01:
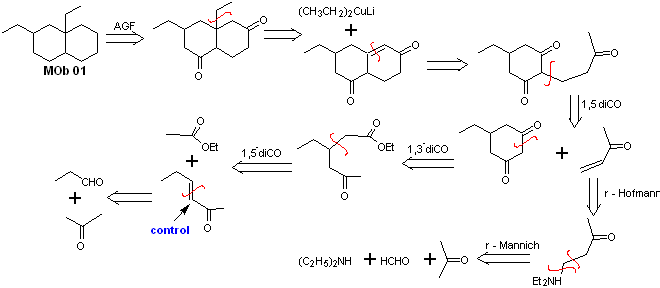
Synthesis of MOb 01 : The condensation between an enolizable aldehyde and ketone normally produces self-condensation or cross-condensation products. This can be avoided, resorting to the strategy of exerting control, in the nucleophile of the ketone, as can be seen in the attached scheme.
On the other hand, when it is required to use vinyl ketones as a substrate in the Michael reaction, it would mean the use of formaldehyde. Unfortunately, this aldehyde, being very reactive in a basic medium, tends to cause polymerization reactions, which drastically lower the yield of the synthesis. For this reason, the Mannich reaction and the Hofmann elimination are adequately combined for vinyl ketones with high yields. .
The Hofmann elimination could be carried out in the same basic medium used for the Michael reaction, so it is not necessary to isolate the vinyl ketone.
However, due to the still limited experience in synthesis, the use of silver oxide in an aqueous medium will be postulated to achieve the elimination of the amine and formation of the vinyl ketone.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 46386
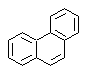
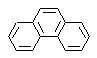
phenanthrene synthesis
(Synthesis Tree Method)
Phenanthrene is a polycyclic aromatic hydrocarbon, which contains three fused benzene rings, which is why it is an isomer of anthracene.
|
|
either |
|
The traditional synthesis methods of phenanthrene, those that involve the formation of cycles and their subsequent "aromatization", are linked to those proposed by Haworth and Bardhan - Sengupta (1932), as will be seen below.
It is also possible to propose other new methods, for Phenanthrene and in general for polycyclic aromatic compounds, based on the appropriate use of the Ullman coupling reactions, the Heck reaction, the Suzuki reaction and the MacMurry reaction, as well as the variants and extensions that present these reactions.
a) Haworth's synthesis:
This method is based on the Friedel-Crafts acylation reaction and has the drawback that the final cyclization to form the third ring fused to naphthalene is not selective, because it is also possible that the closure occurs in the other carbon adjacent to the group that contains the carboxylic function and thus forms an isomer, which due to the reaction conditions turns out to be the minority.
Method A :
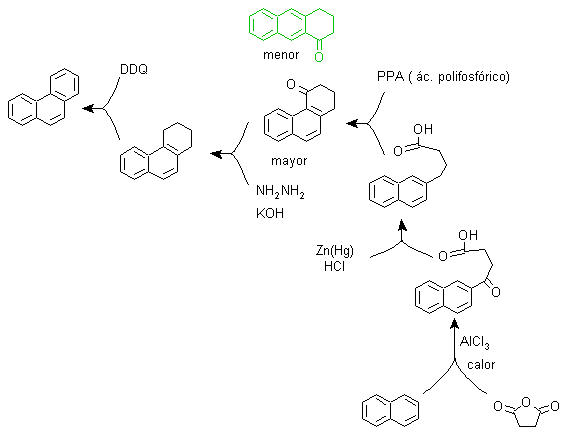
The starting materials are usually naphthalene and succinic anhydride. To ensure that the acylation reaction with the anhydride occurs at carbon 2 (beta) of naphthalene, it is necessary to carry out the reaction at a temperature higher than 60 ºC.
At room temperature, the acylation position will be carbon 1 (alpha), which gives rise to a variant to the method, which, however, are essentially the same reactions that occur and there is also the formation of another isomer that is much less significant than in the first case. Therefore, it is preferably used as the official reaction for the preparation of phenanthrene by the Howorth method.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 32561
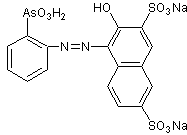
THORINE SYNTHESIS
The analytical applications of thorin, the working parameters, its limitations and prospects, are addressed and explained in sufficient detail in specialized chemical analysis publications such as Analytical Chemistry . A brief summary of them shows the following applications:
In an acid medium : it is used for the quantitative determination of the elements thorium (Th), zirconium (Zr), fluorine (F), hafnium (Hf) and uranium (U).
In a basic medium : The disulfonic acid form of thorine, better known as Thoron, is used in the quantitative determination of lithium (Li).
In a neutral medium : thorin is used as an indicator for the determination of sulfate ions (SO 4 2- ) in aqueous solutions.
Thorin is also used to determine SO 2 in air.
Research Problem.
Given the demand for this reagent for different analytical determinations and particularly for the colorimetric analysis of lithium in brines from the Gran Salar de Uyuni and the low supply of it in the national market, the need arises to synthesize thorine, from simple materials . and affordable in our environment.
With this purpose, the designed synthesis routes are approached from the RETROSYNTHESIS paradigm , being applicable the methods of The Synthesis Sheets, the Disconnection or Synthon Method and the Tree Method. of Synthesis.
In relation to the synthesis of thorin , there is a brief and summarized description of the synthesis of thoron in the literature , according to the proposed direction by Kuznetsov , that is to say, it is indicated that for the preparation of thoron, it is required that the o-aminophenylarsonic acid be diazocopulated with the disodium salt of 2-naptol-3,6-disulfonic acid (salt R) in an acid medium.
Synthesis design for the Thorina .
The design is approached by the method of disconnections or Sintón, which contemplates two stages. The first is related to the retrosynthetic analysis and the second to the synthesis in the direction of what happens in the laboratory, that is, from the starting materials until arriving at the Target Molecule (MOb).
to) Items structural and reactivity to be considered, for the retrosynthetic analysis
Thorin is typically an azo compound, therefore a dye. The preparation of these compounds generally comprises two fundamental reactions, which are: diazoation and diazocoupling (or simply coupling).
On the other hand, all the coupling precursor molecules used for the formation of azo dyes have a common character, that is: an active hydrogen atom linked to a carbon atom.
The following are widely used as precursor molecules (substrates) for coupling: Compounds that have phenolic hydroxyls, such as phenols and naphthols, Aromatic amines, Molecules with enolizable aliphatic ketone groups, and Heterocyclic molecules such as pyrrole, indole, etc. .
In relation to the copulation reaction, certain heuristic principles must be taken into account, namely:
· Phenols couple more easily than amines and members of the naphthalene series more easily than benzene.
· Substituents with negative inductive effect (-I), such as halogens, nitro, sulfa, carboxyl, and carbonyl groups retard coupling.
· An alkyl or alkoxy group in the ortho or meta position with respect to an amino group promotes coupling. And if they are in positions 2 and 5 with respect to the amino group, they are especially good couplers.
· In the benzene series, coupling ordinarily occurs at the para position with respect to the hydroxyl (-OH) or amino (-NH 2 ) group. If the position for is occupied, the link occurs at the ortho position.
· In the naphthalene series, when the amino hydroxyl group is at position 2 ( b ), the reactant is coupled at position 1 ( a ). If the hydroxyl or amino group is in the alpha position, the bond usually occurs at the 4 position; but if position 3 or 6 is occupied by a sulfonic group, the union takes place in the beta position.
· When there are two possible positions of coupling, the position of the bond is frequently decided by the pH of the medium: The coupling occurs in ortho with the amino group when it is carried out in an acid medium and in ortho with the hydroxyl group when it is carried out in a basic medium. .
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 60319
SYNTHESIS OF BENZODIAZEPINES (BDZ)
By. wilbert rivera munoz
This email address is being protected from spambots. You need JavaScript enabled to view it.
These substances produce a wide variety