Synthesis of Fluoroquinolone Antibiotics
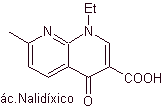
Quinolones belong to a group of synthetic antibacterial agents. The oldest agent in this family, nalidixic acid, used in the early 1960s, has a good spectrum against enterobacteria (limited antibacterial spectrum) but its pharmacokinetics are not very favorable for routine clinical use due to its low bioavailability. in tissues and its short half-life.
For this reason, it was necessary to synthesize new antibacterials of this family to improve the spectrum of activity, the pharmacokinetic profile, reduce adverse effects and the appearance of bacterial resistance. This new group are the so-called fluoroquinolones, generated during the 80s.
|
Many researchers agree that the Gould-Jacobs reaction , is the main basis for the synthesis of the first quinolones for pharmacological use, which occurred in the 1960s, this reaction presents the following sequence:
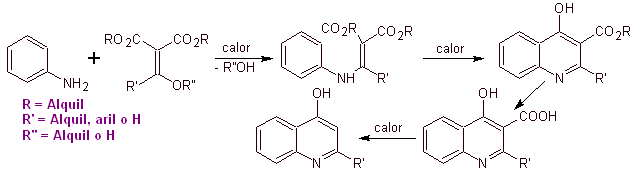
In the following years, fluorine in position 6 and various groups of heterocycles in position 7 have been introduced into the basic ring of benzoquinolones, giving rise to fluoroquinolones with a greater antibacterial spectrum.
Important parts of the methodologies used in these syntheses have been compiled by Leyva S and Leyva E in very good work from a biochemical perspective.
The disconnection method applied to the report of the syntheses under study and the reactions of the first stages of the syntheses are the sole responsibility of the author of this monograph. .
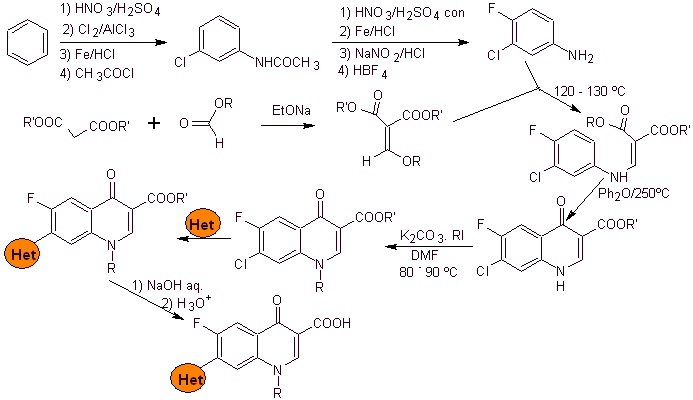
i) Synthesis reported by Koga H. et al.
Retrosynthetic analysis:
Synthesis: It starts from benzene to form 3-chloro-4-fluoroaniline, which reacts with diethyl EMME to produce the corresponding acrylate, which upon heating forms a cyclic compound. This compound in turn is reacted with an alkylating agent and subsequently the nitrogenous heterocyclic compound is introduced, to finally hydrolyze and obtain the target molecule.
Retrosynthetic analysis:
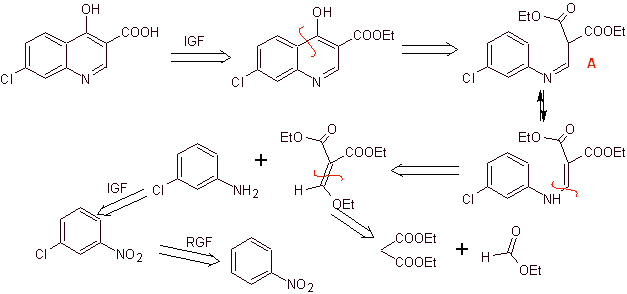
Synthesis: Starting from benzene, to form 3-chloroaniline with diethyl ethoxymethylenemalonate (EMMET) formed from the condensation of ethyl formate and diethyl malonate in a basic medium, to produce compound A, which is subsequently heated in the presence of a diphenyl ether to generate the quinoline cyclic compound, which is readily hydrolyzed to the corresponding acid.
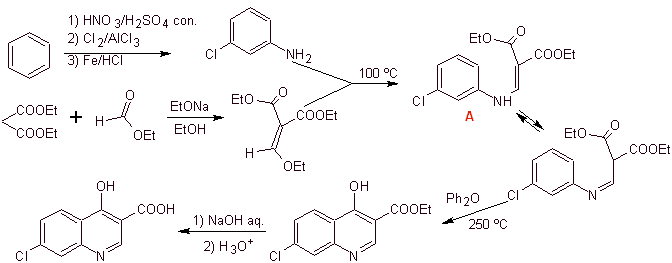
iii) Synthesis reported by Grohe and Zeiler
Retrosynthetic analysis: The CN bond begins to be disconnected and the amine continues to be disconnected, to reach the 1,3-diCO compound, which could be disconnected as such, but the reaction proposed by its authors is respected and diethyl malonate is generated as intermediate, as well as the multihalogenated derivative of benzoyl chloride.
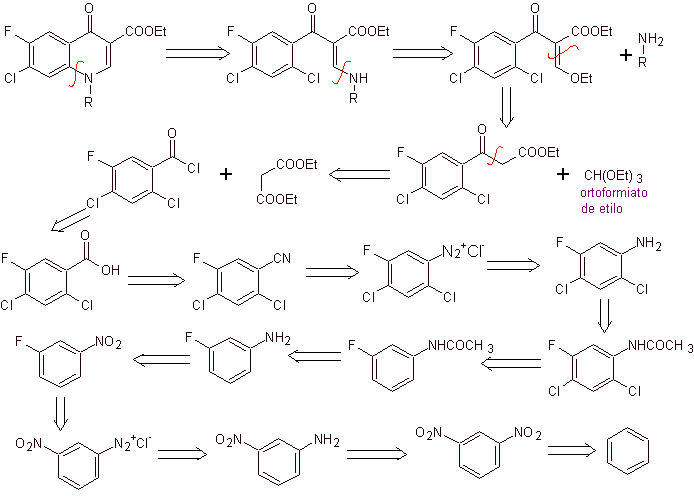
Synthesis. From benzene , ethyl benzoyl acetate can be obtained substituted with fluorine and chlorine in the corresponding positions. This compound is reacted with triethyl orthoformate to produce the corresponding ethoxyalkene, where it is possible to substitute the ethoxy group for an amino group to produce the respective amine, which is cyclized with a strong base to the fluoroquinolone. as can be seen fluorine, chlorine or nitro participate as possible leaving groups in the cyclization reaction. This method has been very versatile, and has been used in the synthesis of N-aryl and N-alkyl fluoroquinolones.
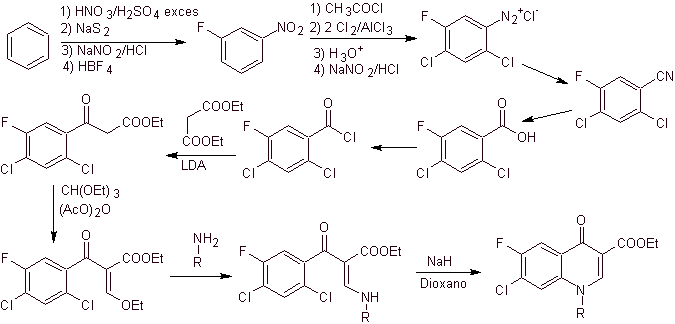
iv) Summary reported by Grohe and Heitzer
Retrosynthetic analysis .
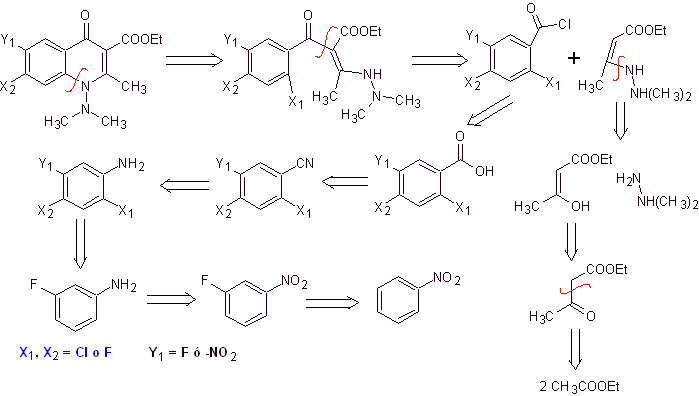
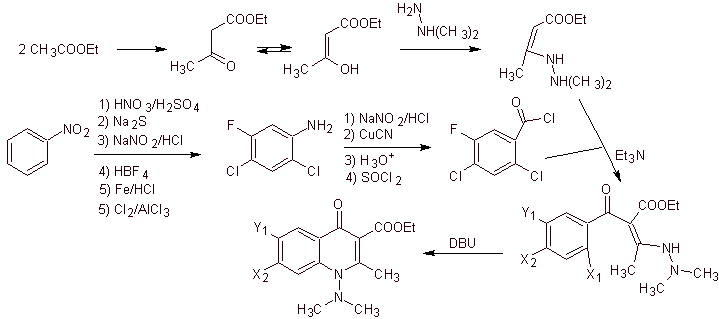
Synthesis. A convergent synthesis route is proposed. One of which starts from the Claisen condensation of two moles of ethyl acetate, to produce ethyl acetoacetate, which is combined with the methylated derivative of hydrazine, which forms the vinylogous diamine, which will be used in the reaction with the derivative of benzoyl chloride, formed from nitrobenzene due to the catalytic action of a tertiary amine.
The compound formed is cyclized by the catalysis of DBU (1,8-Diazabicil[5.4.0]undec-7-en), to produce a fluoroquinolone.
v) Synthesis reported by Torii et al.
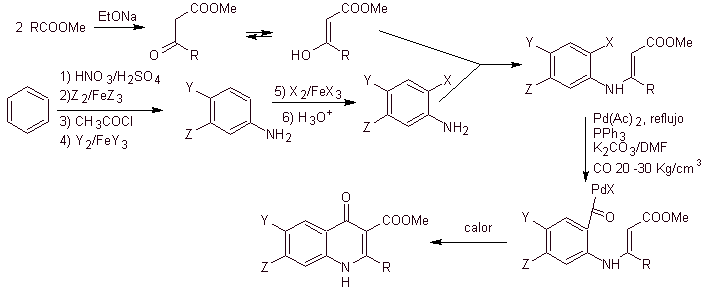
Retrosynthetic analysis. Torii and his collaborators in 1990 have reported the synthesis of fluorinated quinolones by means of a carbonylative cyclization process, catalyzed by Pd. This method is very suitable for the synthesis of fluoroquinolones, substituted in position 2 (R = –Me, -CH 2 COOMe, –COOMe). A retrosynthetic analysis of this method begins by disconnecting at the acyl bond and proposing an organocadmium as a precursor molecule or synthetic equivalent. The rest of the disconnections lead to multisubstituted aniline. with halogens, as follows:
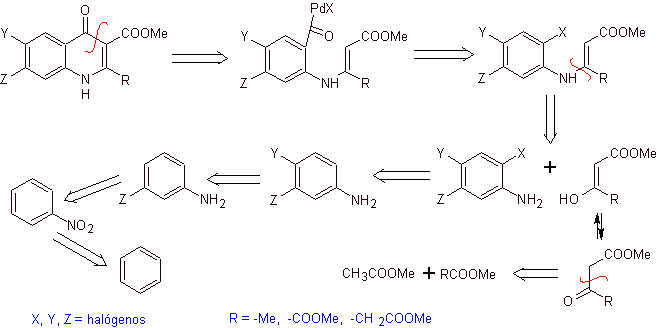
Synthesis. The synthesis design can be sketched again through a convergent route. The first route refers to the formation of ethyl acetoacetate and the second, starting from benzene to multihalogenated aniline, which will cyclize under catalytic carbonization conditions in the presence of palladium. A final heating of the system allows for cyclization and formation of the fluoroquinolone.
vi) Synthesis reported by Miyamoto et al.
Retrosynthetic analysis:
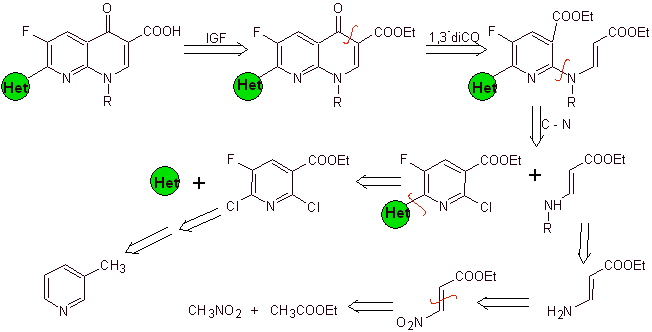
Synthesis. Since the halogens at the 2- and 6-positions of pyridine are highly reactive, it is possible to prepare quinolones by the procedure shown by Miyamoto et al, in which the 2,6-dichloropyridine ester is reacted with a cyclic amine to give an intermediate that is reacted with the vinyl amine, to then cyclize in a basic medium. Hydrolyzed the ester is formed
the fluoroquinolone.
In any case, the formation of the halogenated ester at the positions 2, 6 with chlorine and F in 5. It is the critical part of this synthesis. The multihalogenated pyridine ester can be prepared from 3-methylpyridine (beta-picoline), which is obtained from the distillation of coal tar.
vii) Synthesis reported by Hayakawa
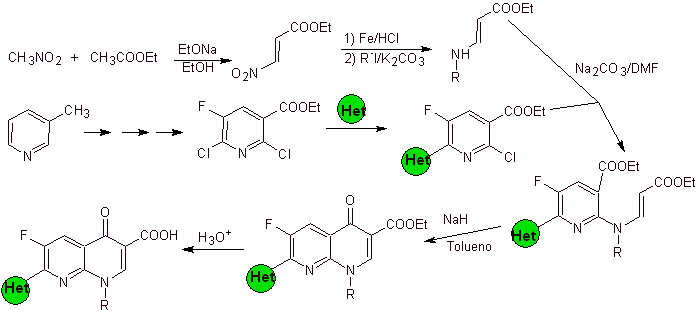
Retrosynthetic analysis: Compounds such as flumequine and ofloxacin have a tricyclic structure. Their synthesis is carried out according to Hayakawa et al., starting from a structure that already contains the nitrogenous heterocyclic ring.
Consequently, the disconnection by the CO bond begins, resulting from the acylation of the benzene ring, and the CN disconnection continues, which leaves a bicyclic structure and EMME (diethyl ethoxymethylene malonate) as synthetic equivalents. The continuity of disconnection depends on the nature of X (-CH 3 , O, S). in the case of being O or S, the disconnection can be simultaneous as indicated. The following operations are IGFs that allow a glimpse of benzene as a starting material.
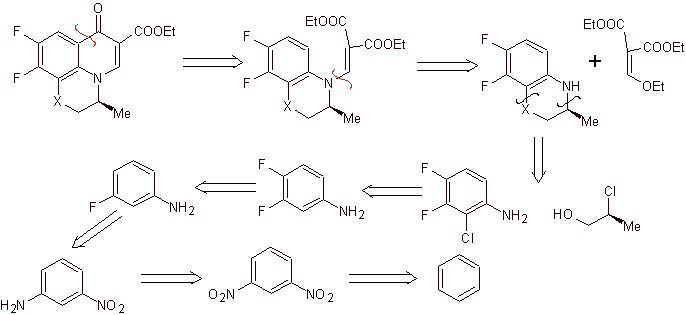
Synthesis. Sandmeyer reactions can be appropriately combined to introduce F and create structural conditions to introduce chlorine. The formation of the heterocycle can be carried out with 2-chloropropanol, formed from the opening of a suitable epoxide. The rest of the reactions that include the use of EMME are already known by the methods previously seen for the synthesis of fluoroquinolones.
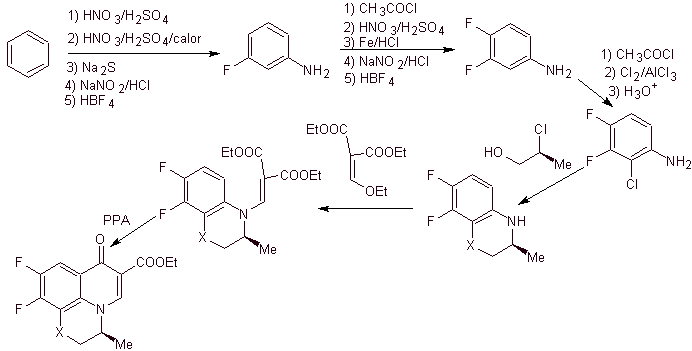
viii) Synthesis reported by Egawa et al.
Retrosynthetic analysis . The tricyclic compound begins to be disconnected by the C-O bond, an aspect that allows us to see that this bond was the result of an intramolecular cyclization of a –OH compound with the F of benzene. A second CN disconnection generates an EMME amino precursor molecule. The following disconnections are well known.
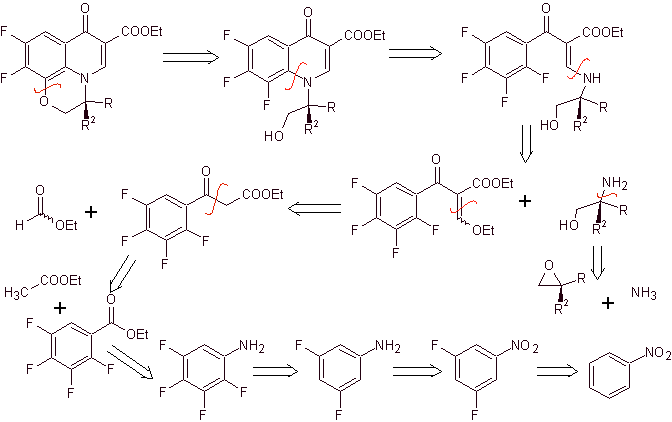
Synthesis. The exhaustive fluoridation, by indirect methods of benzene, is achieved first using nitrobenzene as starting material, then transforming it into an amine or amide, in order to occupy the other positions.
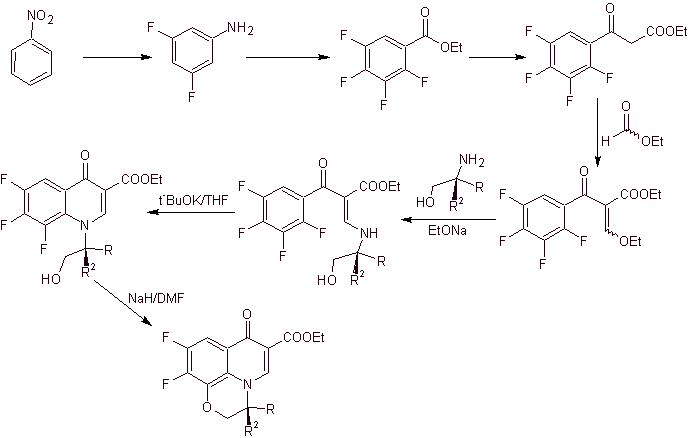
ix) Synthesis reported by Matsumoto et al.
Retrosynthetic analysis.
Synthesis: In the procedure reported by Matsumoto et al. the Gould-Jacobs reaction was again used; starting from a dichlorinated nitropyridine, the intermediates were synthesized, first with
The third cycle is achieved using EMME and alkylating the amine with ethyl iodide, finally hydrolyzing the amide and ester groups.
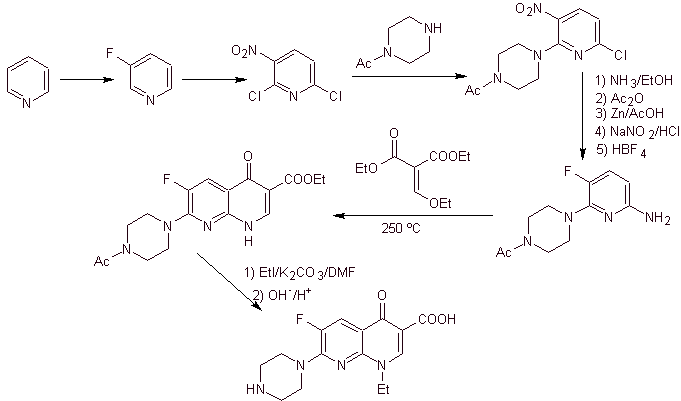
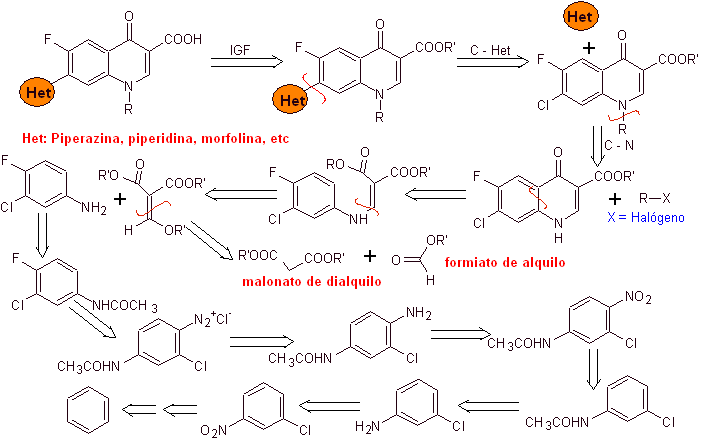
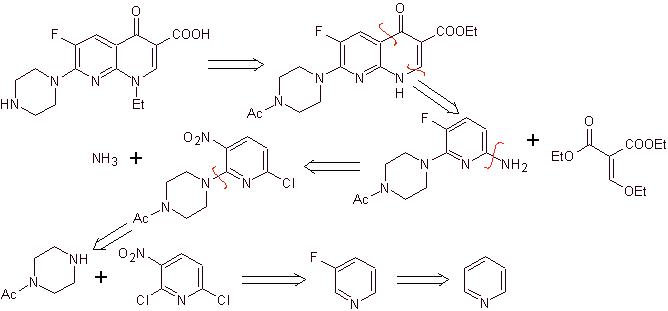
X) Synthesis reported by Chu et al.
Retrosynthetic analysis: It begins by disconnecting the CN bond of the heterocycle and continues with the CN of the vinylogous amine. In EMME, it is disconnected by the double bond, to produce ethyl formate as a precursor molecule, the next disconnection is of the 1,3-diCO type, which will link us to the Claisen condensation. Then the resulting aromatic ester is subjected to various IGFs, until reaching nitrobenzene as starting material
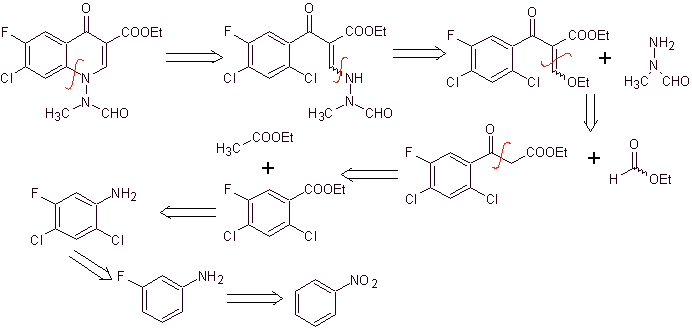
Synthesis. One can start from nitrobenzene, and with the necessary reactions, reach the ethyl benzoati suitably substituted with F and Cl, from which the Claisen condensation is used followed by another Knoevenagel condensation with ethyl formate. This intermediate is then reacted with N-formyl-N-methylhydrazine and with the addition of NaH, cyclization is achieved to arrive at the final compound.
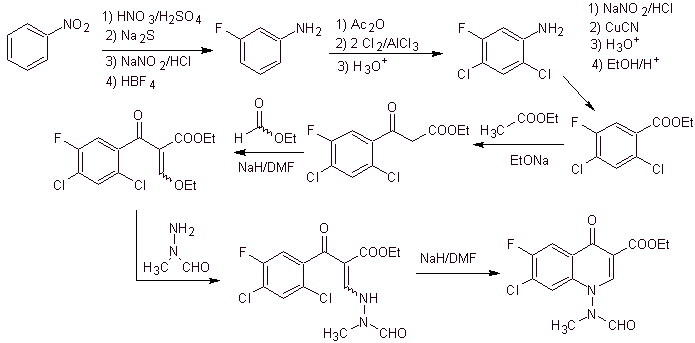
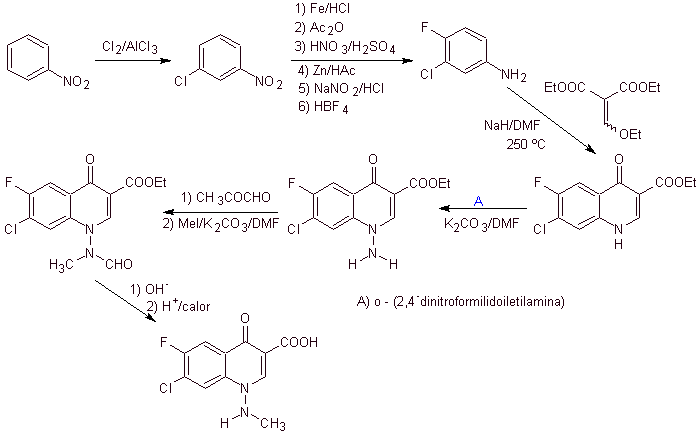
xi) Synthesis reported by Wenthand et al.
Retrosynthetic analysis Quinolones with a substituted amino group at position 1, such as amifloxacin, can be synthesized following the methodology described by Wentland. For this reason, disconnections are initiated by the N-C link. and then it continues with the NN bond and the closure of the ring, the Gould-Jacobs reaction follows.
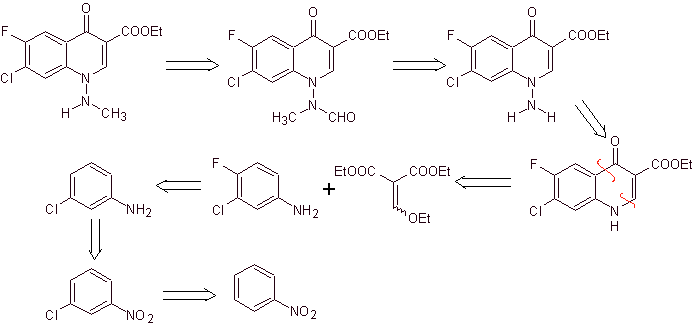
synthesis . In this case, the amino group of the quinolone is reacted with a reagent that transfers another amino group, which is subsequently converted to a formamide derivative in order to later transform it to the compound 1-(N-methyl)-7-chloro -6-fluoro-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid.