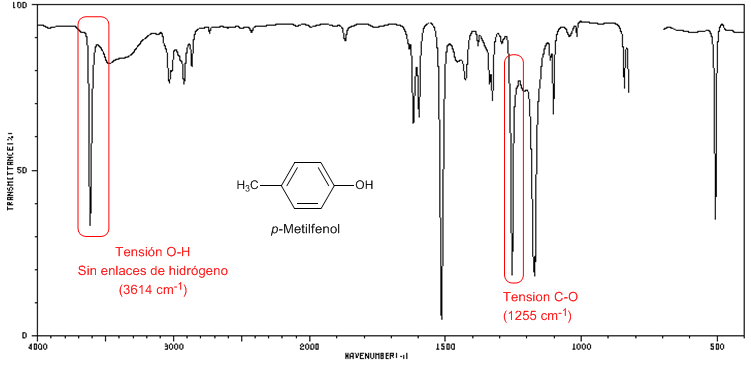
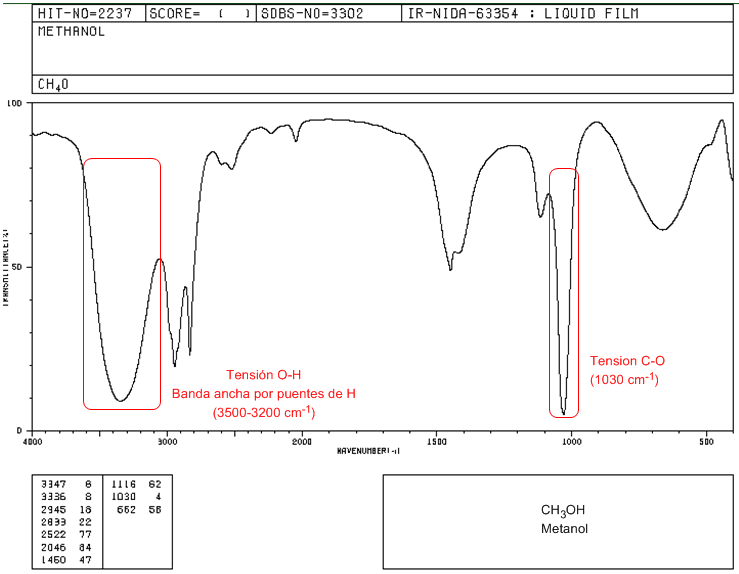
♦ OH tension: Broad band from 3500 to 3200 cm -1 . In the absence of hydrogen bonding it appears as a sharp peak at 3650-3600 cm -1 .
♦ CO tension: Band between 1250-1000 cm -1 . It makes it possible to distinguish between primary (1050 cm -1 ), secondary (1100 cm -1 ), tertiary (1150 cm -1 ) and phenols (1220 cm -1 ) alcohols.
IR spectrum of methanol
In the spectrum of methanol we can observe the very wide OH tension band, due to the formation of hydrogen bonds. The CO tension band comes out at a low wave number (1030) because it is an alcohol without substituents.
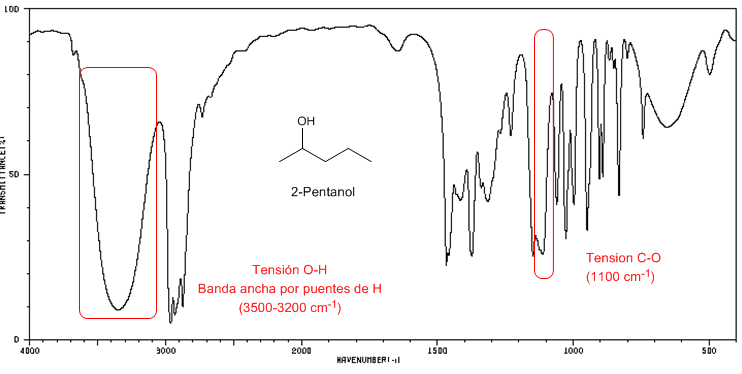
IR spectrum of 2-Pentanol
Observe the displacement of the CO band towards a greater number of waves with respect to methanol.
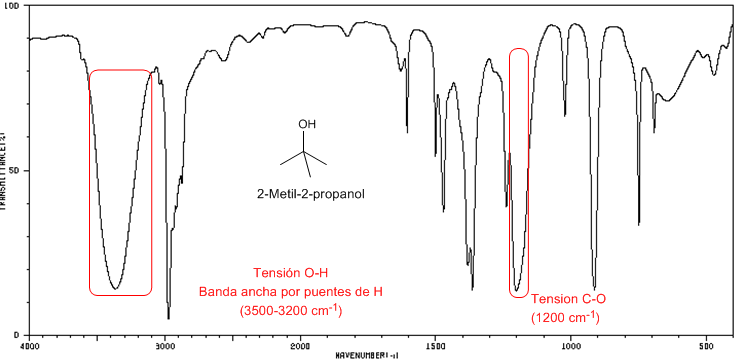
IR spectrum of 2-methyl-2-propanol
Tertiary alcohols have the CO band shifted to higher frequencies than primary and secondary alcohols.
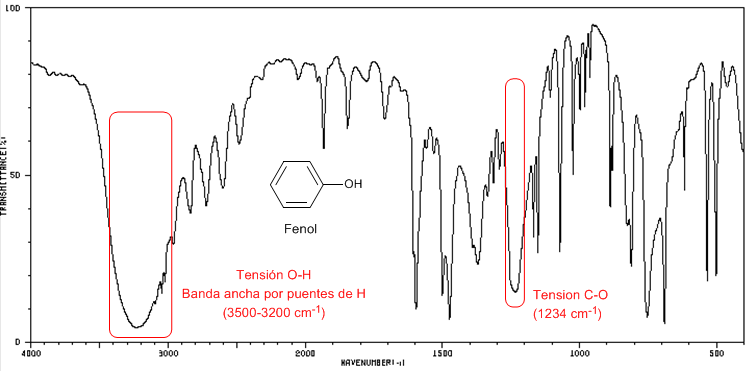
IR Spectrum of Phenol
Phenol presents a CO absorption band above 1200 cm -1
IR spectrum of p-Methylphenol in CCl 4
The following spectrum shows the OH stretch band in the absence of hydrogen bonding.