For a molecule to absorb infrared radiation, two conditions must be met:
- The frequency of the radiation (photon) must be adequate to allow the transition between vibrational states. In other words, the frequency of the radiation must coincide with the natural frequency of the vibrational movement.
- A molecule only absorbs infrared radiation when its dipole moment interacts with the electric field of the wave, varying in phase with it. As is logical, this coupling is only possible if the frequencies of the radiation and the vibration of the link coincide. For this reason, apolar molecules do not absorb in the infrared and less polar molecules give rise to very weak absorptions.
- Infrared spectroscopy selection rule: "only those bonds whose vibration causes a change in the dipole moment of the molecule absorb in the infrared".
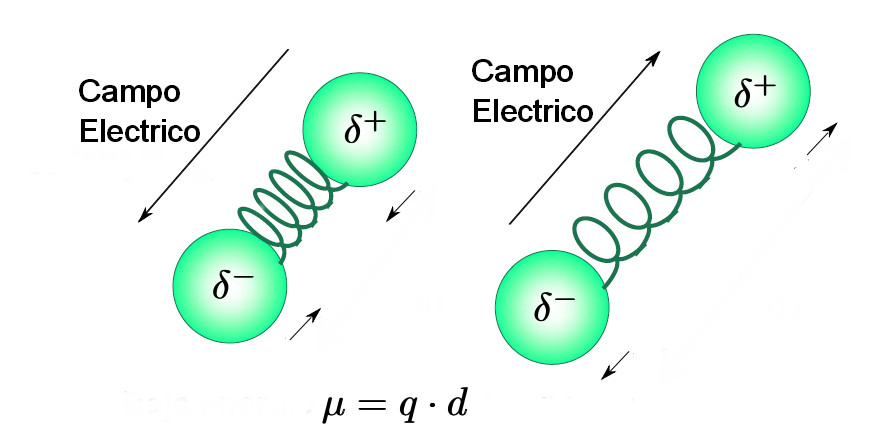
- Furthermore, the larger the dipole moment variation during vibration, the more intense the absorption band in the spectrum. Vibrations of C=O, OH, NH bonds give rise to intense bands and vibrations of triple bond tension are not observable in symmetric alkynes or trans alkenes with equal chains.