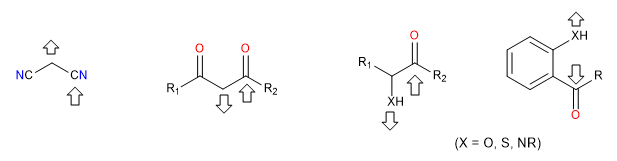
i) Doubly electrophilic reagents
They are reagents that have two positions with strong positive polarity to which nucleophiles attack. These reagents form cycles when confronted with others that are doubly nucleophilic. These are dicarbonyls, alpha, beta-unsaturated, alpha-halogenated carbonyls.
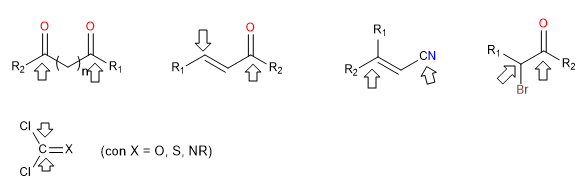
ii) Doubly nucleophilic reagents
They are reagents that have two nucleophilic centers, generally atoms with lone pairs, although they also have double nucleophilic character, triple bonds and aromatic rings.
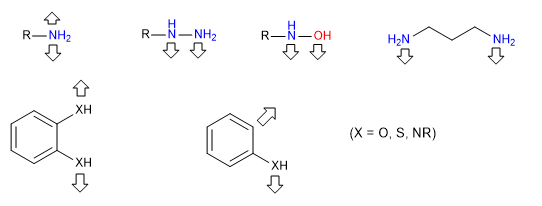
iii) Electrophilic reagents - nucleophiles.
In the synthesis of rings we can also use reagents that combine both electrophilic and nucleophilic centers.