What might be called the “zero rule” for heteromonocycle numbering calls for locant 1 to be assigned to the highest priority heteroatom according to Table 1. Numbering then proceeds such that the lowest possible locants are given to:
1.- All heteroatoms, together.
2.- All the heteroatoms, taking into account the priorities of table 1.
3.- The indicated hydrogen(s)
4.- The atom(s) of the heterocycle carrier(s) of the main function that is named as a suffix (it is only one, according to the substitutive nomenclature, for it can be repeated).
5.- Substituents not belonging to the Hantzsch-Widman system, as a whole (that is, not oxa, aza, etc., but methyl, dihydro, hydroxy, etc.)
6.- Substituents not belonging to the Hantzsch-Widman system, in alphabetical order.
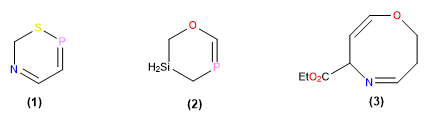
Compound 1 is named 6H-1,5,2-thiazaphosphinine . After assigning locant 1 to S, it is numbered towards P, leading to the sequence of locants 1,2,5 (in increasing sense), which takes precedence over 1,3,6 which would arise if numbered towards the N (Standard 1).
In the name, the prefixes are cited according to the order of priority of table 1; their locants are all grouped in front, in the same order that the prefixes are mentioned.
The symmetrical situation of the three heteroatoms of compound 2 makes rule 1 ineffective, so in this case rule 2 decides. The name of the heterocycle is 5,6-dihydro-4H-1,3,5-oxafosfasiline.
The name of heterocycle 3 is ethyl 3,6-dihydro-2H-1,5-oxazocine-6-carboxylate . Once the number 1 is given to O, we use the norm 3 for what decides the indicated hydrogen. C-6 is not taken as indicated hydrogen since from oxygen it would occupy position 4.
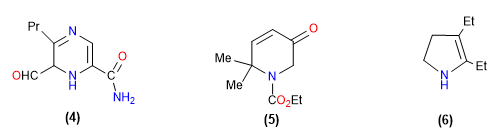
By giving the name of compound 4 , it can be seen that norms 1 and 2 do not decide; there being no hydrogen indicated, the numbering is determined by the presence of a main function (rule 4): of the two present, it is the amide. Thus, the numbering is done by giving the carbon carrying the amide group the lowest possible locant, which is 2. The name turns out to be 6-methanoyl-5-propyl-1,6-dihydro-1,4-diazine-2 -carboxamide .
The main function of heterocycle 5 is the ester, whose locant is 1 by the “zero norm”. No hydrogen indicated, since the ketone function is supposed to have been introduced after a formal double hydrogenation of the pyridine (from three ring double bonds to just one). Rule 5 requires numbering clockwise, and the name of the compound is: ethyl 2,2-dimethyl-5-oxo-1,2,5,6-tetrahydropyridine-1-carboxylate . Counterclockwise numbering would lead to a larger sequence of locants.
Finally, the simple heterocycle 6 has four substituents (diethyl and dihydro), whose overall numbering is identical in both directions. In such cases, and in the absence of other accidents regulated by previous rules, rule 6 applies. In alphabetical order, ethyl takes precedence over hydro, which leads to the following name: 2,3-diethyl-4,5-dihydro-1H -pyrrole.
In short, the indicated hydrogens are so important (rule 3) that they have the highest numbering priority after the heteroatoms. Hydro prefixes are handled as if they were substitute prefixes, and are even included in the sequences of their locants for the purpose of choosing the correct numbering sense (see compound 5). However, indicated hydros and hydrogens are used as non-separable particles of the name, and thus are mentioned just before the name of the heterocycle.