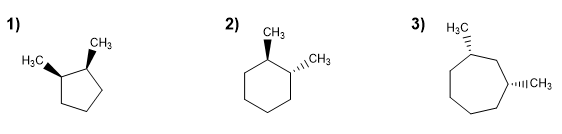
Using cis/trans notation to indicate stereochemistry, name the following cycloalkanes.
SOLUTION:
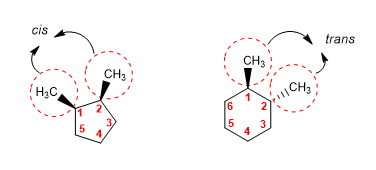
The cis/trans notation allows to indicate the spatial position of a substituent with respect to another. It is used in cycloalkanes and alkenes.
When two substituents are towards the same plane of the molecule (both wedged or dashed), they are said to be arranged cis.
When the substituents go to opposite sides (one with a wedge and the other with a dashed line), they are said to be trans.
Molecule 1.
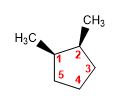
cis-2,3-Dimethylcyclopentane
Molecule 2.
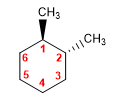
trans-1,2-Dimethylcyclohexane
Molecule 3.
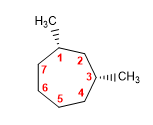
cis-1,3-Dimethylcycloheptane
![]() Note that the cis/trans notation is always written in lowercase italics.
Note that the cis/trans notation is always written in lowercase italics.