Pictet Spengler is a Mannich type reaction. In the first stage, the Mannich electrophile is formed by reaction of the amine with an aldehyde in a hydrochloric acid medium. In the final stage, the cyclization takes place by attack of the benzene on the Mannich electrophile. The isoquinoline is obtained after a double oxidation.
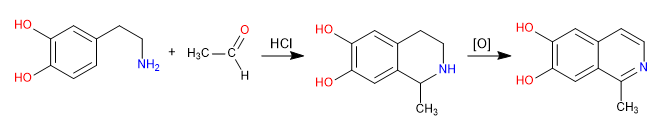
Mechanism:
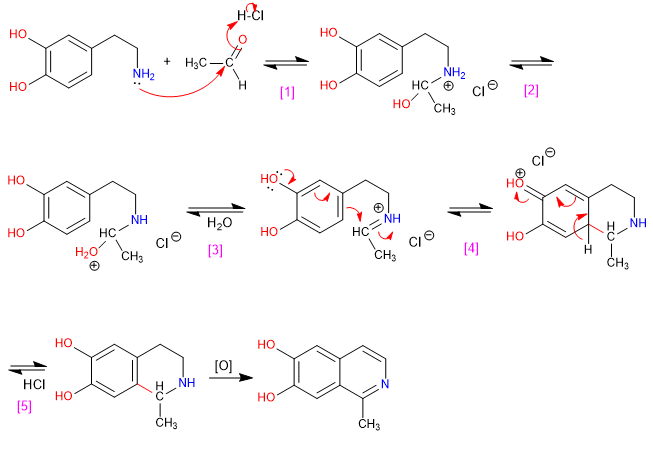
, , Formation of the immonium salt.
Cyclization by attack of benzene on salt.
Rearomatization of the ring.
Oxidation