chemistry click
“Click chemistry” is a term that was introduced by KB Sharpless in 2001 to describe reactions that are high-yield, broad in scope, create only by-products that can be removed without chromatography, are stereospecific, easy to perform, and can be performed in easy-to-remove ways. . or benign solvents.This concept developed in parallel with interest within the pharmaceutical, materials, and other industries in the ability to generate large libraries of compounds for research in discovery research.Several reaction types have been identified that meet These criteria thermodynamically favored reactions that specifically lead to a product, such as nucleophilic ring-opening reactions of epoxides and aziridines, non-aldol-type carbonyl reactions, such as hydrazone and heterocycle formation, additions to carbon-carbon multiple bonds, such as oxidative formation of epoxides and Michael additions, and cycloaddition reactions.
For example, the azide-alkyne cycloaddition shows that it meets many of the prerequisites. Many of the starting monosubstituted alkynes and organic azides are commercially available, many others can be easily synthesized with a wide range of functional groups, and their cycloaddition reaction selectively gives 1,2,3-triazoles.
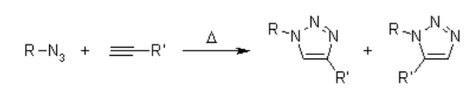
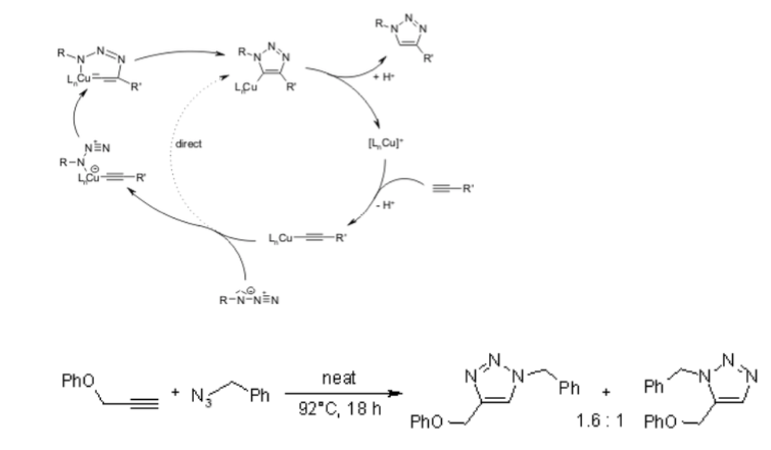
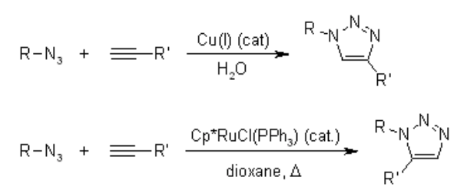
Unfortunately, the huisgen 1,3-Dipolar cycloaddition of alkynes to azides requires elevated temperatures and often produces mixtures of the two regioisomers when asymmetric alkynes are used. In this sense, the classical 1,3-dipolar cycloaddition fails as a true click reaction. A copper-catalyzed variant that follows a different mechanism can be performed under aqueous conditions, even at room temperature. Furthermore, while the classical 1,3-dipolar Huisgen cycloaddition often gives mixtures of regioisomers, the copper-catalyzed reaction allows the synthesis of the 1,4-disubstituted regioisomers specifically. In contrast, a ruthenium-catalyzed reaction developed later gives the opposite regioselectivity to the formation of 1,5-disubstituted triazoles. Therefore, these catalyzed reactions fully meet the definition of click chemistry and have focused on the azide-alkyne cycloaddition as a prototype click reaction.
Mechanism of the 1,3-dipolar Huisgen Azide-Alkyne cycloaddition:
To know the mechanism, one should read about the 1,3-dipolar cycloaddition also present in many ozonolysis. This reaction is highly exothermic, but the high activation barrier is responsible for a very low reaction rate, even at elevated temperatures. Another drawback is the formation of regioisomers, since the two possible HOMO-LUMO interactions of the substrates are closely related in terms of energy. Thus, thermal reaction often gives approximately 1:1 mixtures of both the 1,4- and 1,5-substituted regioisomers.