• Tension C(sp 2 )-H: 3100 -3000 cm -1
• Tension C=C: 1600 cm -1
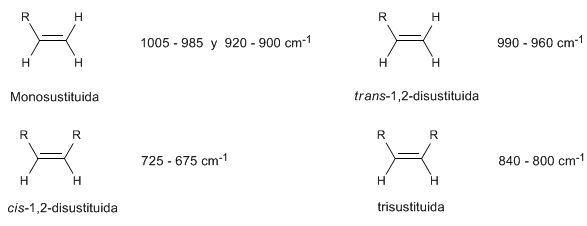
• Out-of-plane bending (oop) of the C=CH bond: 1000 - 650 cm -1 . This type of band allows to know the degree of substitution of the alkene.
IR spectrum of 1-pentene
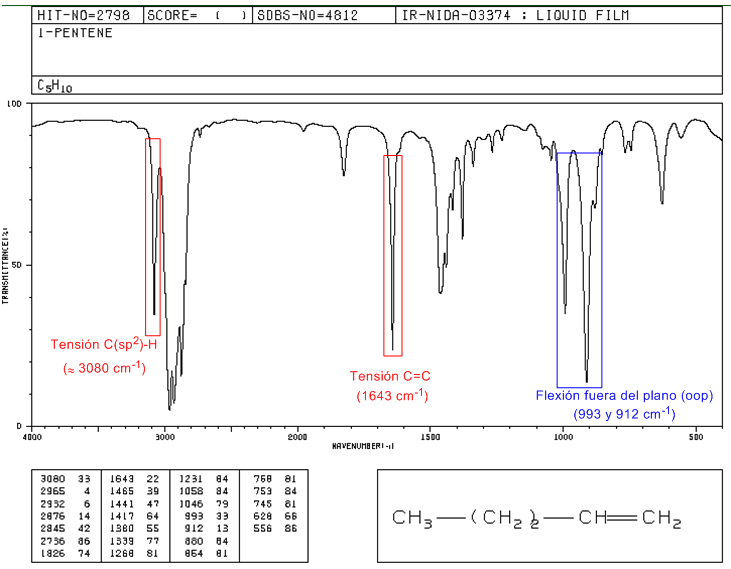
In monosubstituted alkenes, such as 1-pentene, out-of-plane CH bends produce two bands located at 1005-985 and 920-900 cm -1 .
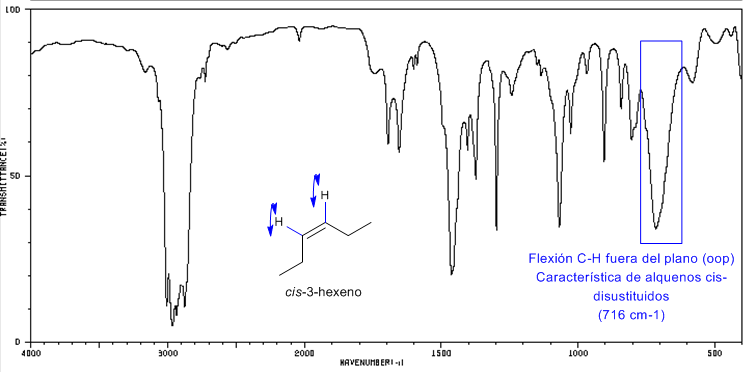
IR spectrum of cis-3-hexene
Cis-disubstituted alkenes present an out-of-plane CH bending band that allows them to be distinguished. This band appears between 725-675 $cm^{-1}$
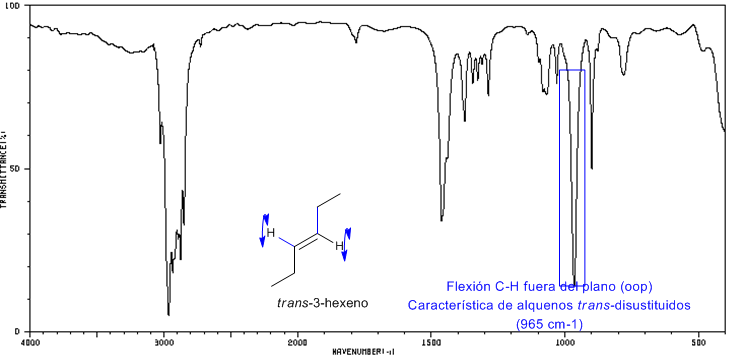
IR spectrum of trans-3-hexene
The trans-disubstituted alkenes present a strong absorption band between 980-965 $cm -1 which allows their identification. Note the complete absence of the C=C tension band at 1600 cm^{-1}$ due to the lack of polarity.
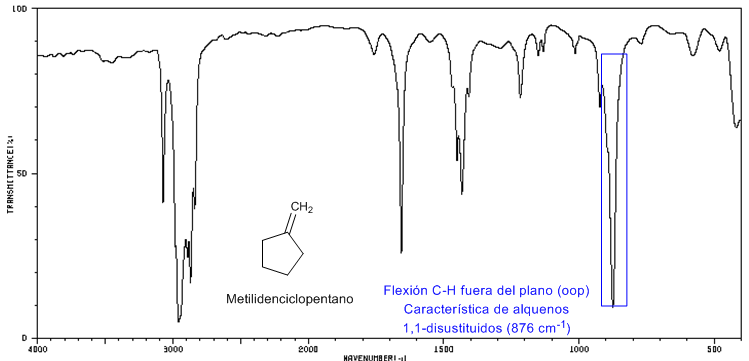
IR Spectrum of Methylenecyclopentane
Methylenecyclopentane is an example of a 1,1-disubstituted olefin and presents a very strong out-of-plane CH bending band, located between 900-880 $cm -1 .
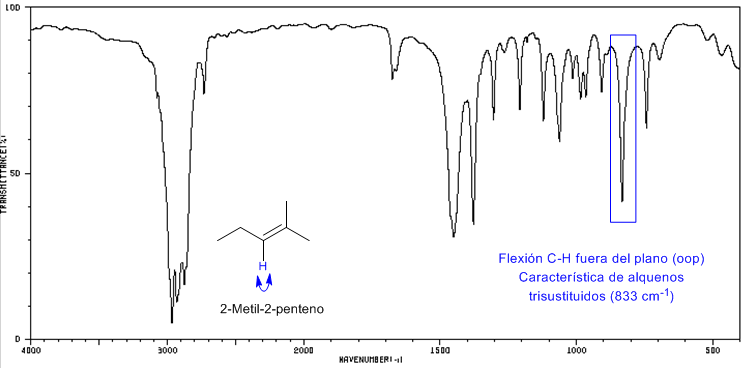
IR spectrum of 2-methyl-2-pentene
2-Methyl-2-pentene is a trisubstituted alkene that exhibits a strong absorption band between 840-800 cm -1 due to CH(oop) bending.
Summary of Out-of-Plane (oop) CH Pushups
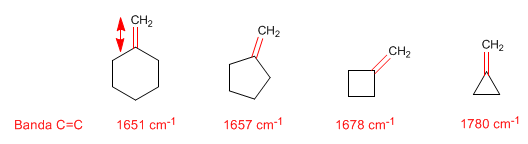
Ring strain: exocyclic C=C bonds
The vibration frequency of the C=C bond increases with decreasing ring size. Thus, methylidenecyclohexene absorbs at 1561 $cm^{-1}$, a typical value for an alkene, while methylidenecyclopropane absorbs at 1780 $cm^{-1}$.
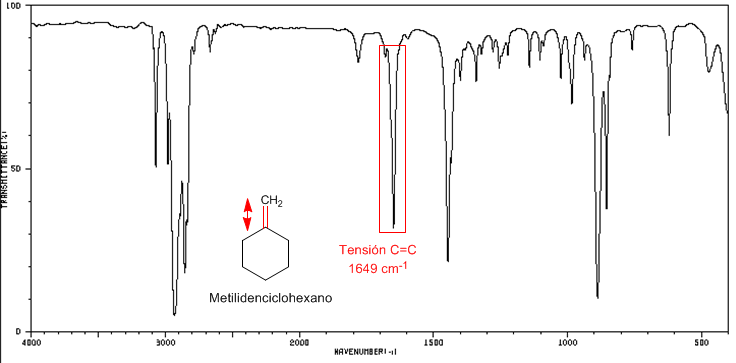
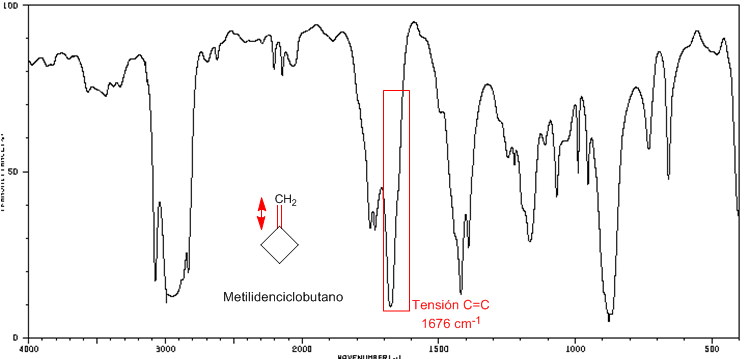
I include the spectra of methylenecyclohexane and methylenecyclobutane to see these displacements, from the C=C tension band, towards a greater number of waves as the cycle tension increases.
IR spectrum of methylidenecyclohexane
IR spectrum of methylidenecyclobutane
Ring Strain: Endocyclic C=C Bonds
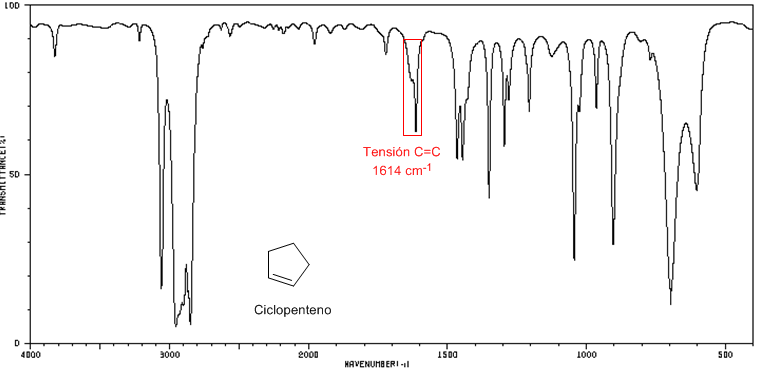
As the size of the ring decreases, the tension band of the C=C bonds moves towards a smaller number of waves. The exception of cyclopropene is attributed to the coupling between the tension vibrations of the C=C and CC bonds. This coupling does not occur in cyclobutene because the C=C and CC bonds are perpendicular to each other.
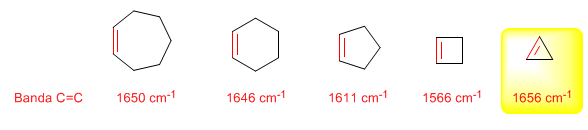
IR spectrum of cycloheptene and cyclopentene