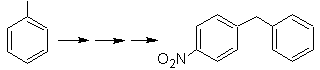SYNTHESIS OF AROMATIC COMPOUNDS II
(Synthesis Tree Method)
Although one of the first problems to be solved in the synthesis of multi-substituted aromatic compounds is the control of the orientation effects and the formation of undesired isomers, it is also important to study the reactivity of the arenes, since at some point In the sense of the presence of aliphatic groups in the aromatic compound, many times, they present characteristics and reactivities, typical of the type of organic compound to which they belong and the particular ones that result from the mutual interaction of the aliphatic and aromatic groups.
On this purpose, it is based, the synthesis of molecules No. 20 to 27, this time from specified materials, so the question is presented as follows: What are the reactions that Justify the following transformations?
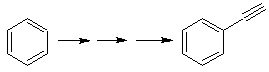
Mob 20 solution.
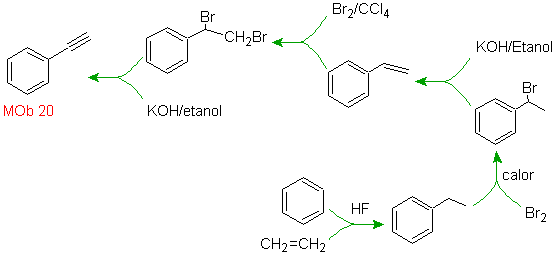
We know that there is no possibility of the acetylide ion acting directly on benzene, therefore the triple bond is obtained from an alkyl group
vec-dibrominated, which is obtained by brominating styrene, previously obtained by dehydrobrominating of a benzyl halide formed by a bromination by the free radical mechanism on ethylbenzene
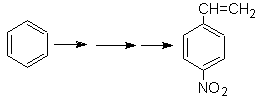
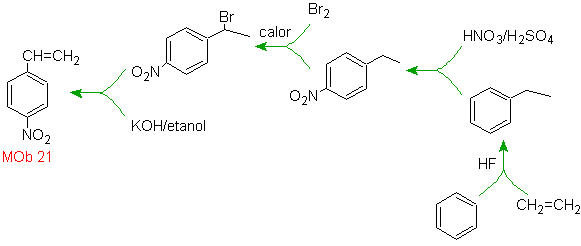
Solution Mob 21.
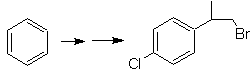
The para nitrostyrene cannot be obtained by direct nitration of styrene, because the ethenyl group attached to the ring is unstable under nitration conditions.
As such, the precursor molecule will have a group that is easy to dehydrobrominate. This precursor is obtained by radical bromination of the ethyl group linked to the benzene ring, which was previously nitrated mainly in the para position.
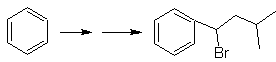
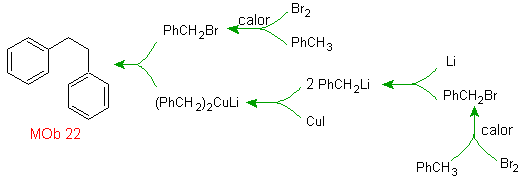
Solution MOb 22.
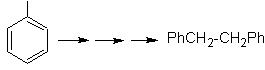
The symmetry of the molecule allows us to think of a strategy that takes into account the Corey-House reaction. It is also a good route if you use the
PhCH 2 CH 2 Cl, over the benzene or an acylation with PhCH2COCl and subsequent reduction of the carbonyl group by Clemmensen reduction are taken into account.
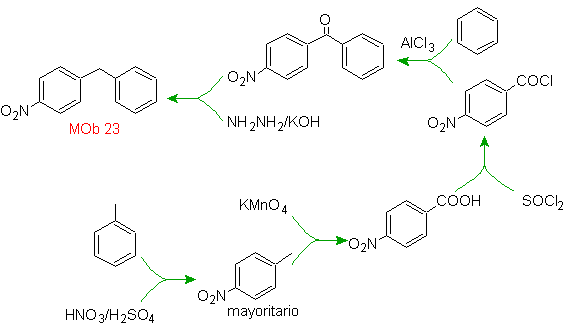
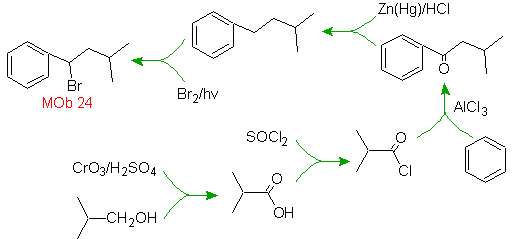
Mob solution 23.
The precursor molecule can be a ketone, the carbonyl group of which is reduced to methylene by the Wolf-Kischner reductant. In this way, the presence of Zn in an acid medium that would affect the nitro group is avoided. Another precursor molecule can be the following halide: O 2 N-PhCH 2 Cl that acts on a benzene molecule.
The bromine position in the precursor molecule is the typical allylic position, which is obtained by radical bromination of the corresponding carbon skeleton.
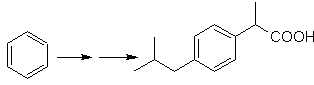
The alkyl group on the benzene ring cannot be obtained from corresponding halide, because transposition would occur. Then, acylation and subsequent reduction of the carbonyl group are used, with zinc amalgamation in an acid medium (Clemmensen reduction
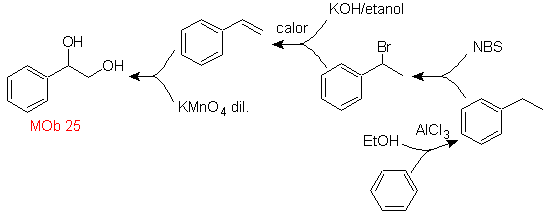
MOb 25 solution.
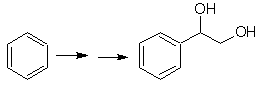
The diol of this molecule can be obtained by a selective hydroxylation of styrene, as
precursor molecule. Styrene is formed from dehydrobromination, as in previous cases.
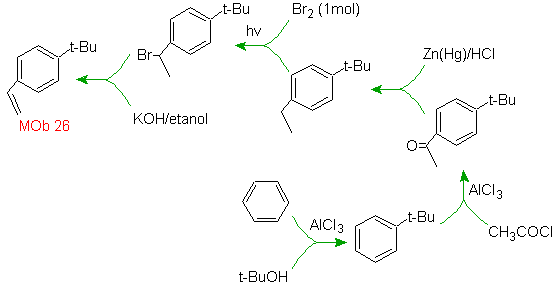
Solution MOb 26.
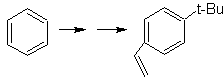
The precursor molecule indicates that a possible route takes into account the formation of the ethenyl group from a halide and takes advantage of the bulky group of t-Butyl, to introduce the acyl group carrying the ethyl group in the para position.
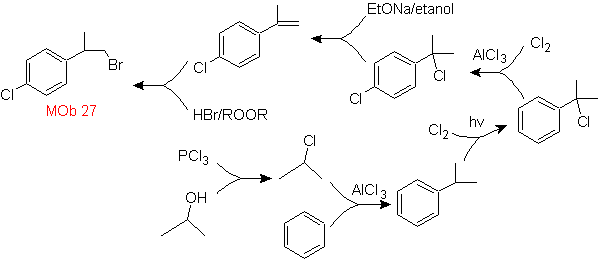
Mob solution 27.
The strategy involves defining the inclusion of bromide in a precursor molecule that does not allow the formation of any isomer. The least substituted alkene is the one that is necessarily formed by a dehydrohalogenation, from a group formed by Halogenation of radicals.
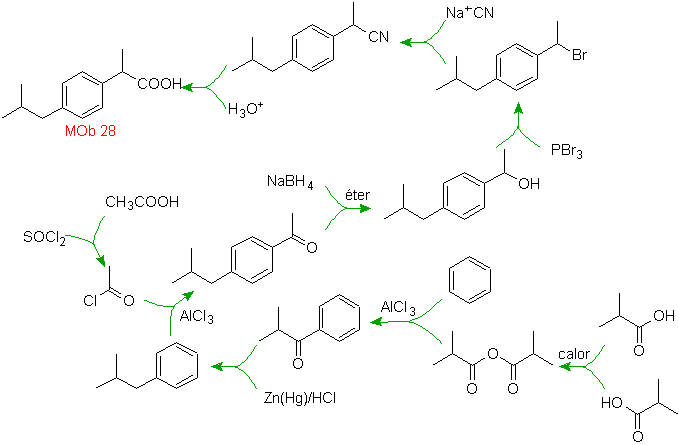
Solution MOb 28.
The location of the carboxylic group allows us to propose its formation from the hydrolysis of the –CN group, the latter is introduced into aliphatic molecules generally by substitution of a halide, which in turn comes from an alcohol. Formed by reduction of a ketone carbonyl.
The subsequent steps are linked to Friedel-Crafts acylation, combined with the reduction of C=O by the Clemmensen method.
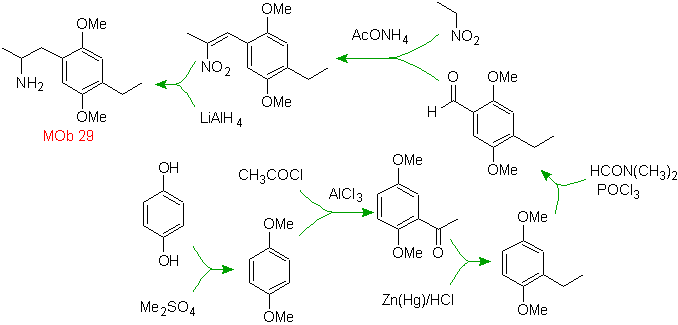
Solution MOb 29.
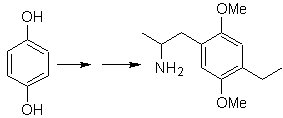
The presence of the amino group in the alkyl residue of the arene, makes us think of the nitro group as its precursor and due to the distance from the benzene ring, it can be proposed that it is formed from the nitro - alpha-beta unsaturated, result of condensation of the aldol type in a basic medium with a –CHO group attached to the benzene ring. This formyl group is inserted into the ring by with a disubstituted formamide and phosphorus oxychloride, known as the Vilsmeier-Haack reaction. Method that can only be applied to activated aromatic substrates. To introduce the ethyl group by Friedel-Crafts Acylation, it is necessary to previously transform the –OH groups of the starting material into methyl ether.