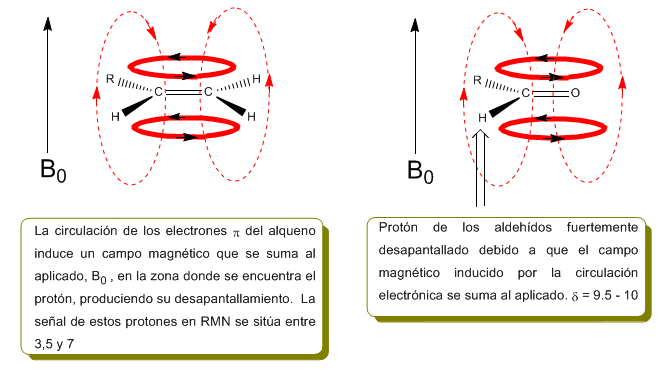
The protons close to double bonds and aromatic rings are especially unshielded due to the magnetic field induced by the electronic currents of these systems. The induced field is added to the applied one, producing a displacement greater than expected.
In the following image we can see the electronic circulation (curves in bold) and the induced magnetic field (dashed lines) for an alkene and a carbonyl. Observe how in the region of the proton the induced magnetic field has the same direction and direction as the applied one.
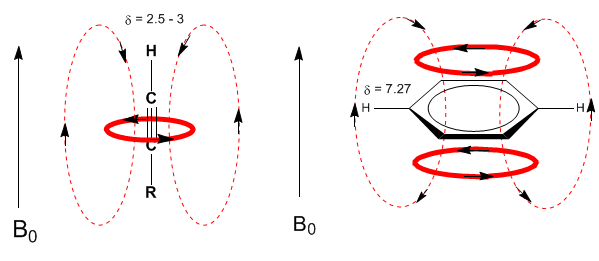
A similar situation is observed in the case of benzene. However, in alkynes, electronic circulation induces a magnetic field that opposes the one applied in the proton area. The acetylenic hydrogens are shielded with signals in the NMR spectrum at low offsets.
Next, I add some spectra of alkenes, alkynes, and aromatics.
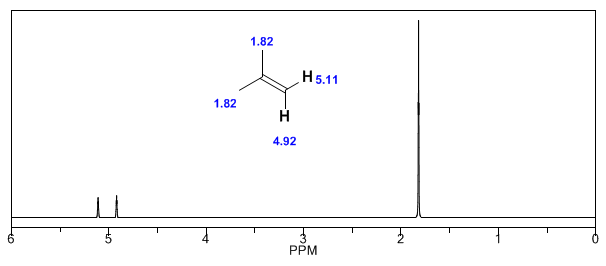
Observe how the induced fields considerably increase the displacements of the olefinic proton, the allylic positions being also affected.
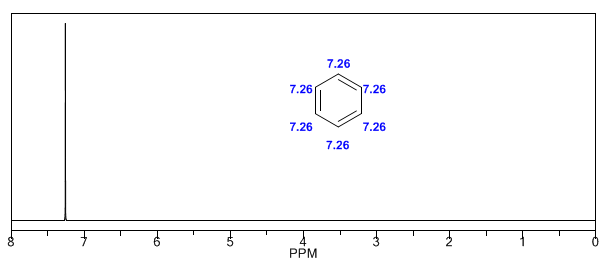
The aromatic hydrogens are strongly unshielded due to the field induced by the ring currents.
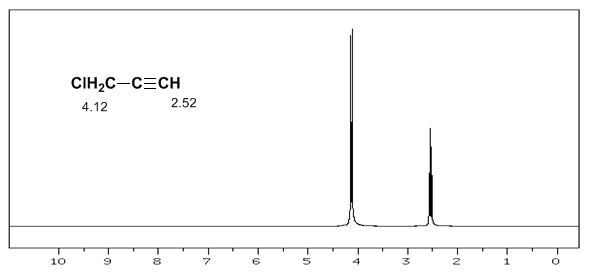
The acetylenic hydrogen has a low displacement, because the currents produce a magnetic field that opposes the applied one.