The sugars in solution are found mostly in a cyclic form, called a hemiacetal. The hemiacetal is obtained by attacking one of the hydroxyl groups of the chain on the carbonyl. The cycles formed are of five or six members.
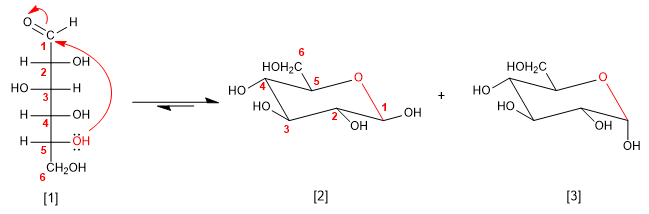
D-Glucose
b -D-Glucopyranose
a -D-Glucopyranose
To draw the hemiacetal we place the -OHs that are on the right in the Fischer projection downwards in the chair, while those on the left are oriented upwards.
The -OH at position 1 (-OH attached to the anomeric carbon) can take two orientations that give rise to alpha (-OH down) and beta (-OH up) anomers. On the other hand, the -CH 2 OH that starts from carbon 5 will be oriented upwards in the D series sugars, while it will be oriented downwards in the L ones.
Glucose can also cyclize as a 5-ring (furanose), by attack of the 4-position hydroxyl on the carbonyl.
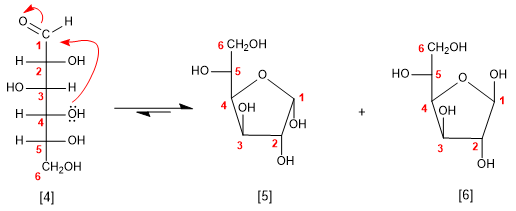
D-Glucose
a -D-Glucofuranose
b -D-Glucofuranose
Although the hemiacetal form is predominant in the aqueous medium, it exists in equilibrium with a small fraction of open molecules. Thus, the reactions that act on the carbonyl group (oxidations or reductions) eliminate the open form from the medium, causing the equilibrium to shift to the left, producing the opening of the hemiacetals.
The pyranose form is more stable than furanose in the case of glucose
Following the rules indicated above, we are going to cycle the arabinose molecule. Attack from the 4-position hydroxyl generates a 5-membered hemiacetal (furanose).
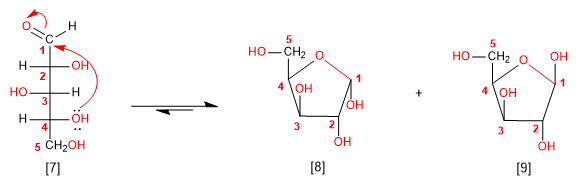
D-Arabinose
a -D-Arabinofuranose
b -D-Arabinofuranose
We can also cyclize arabinose from the hydroxyl at position 5, generating 6-membered cycles (pyranose)
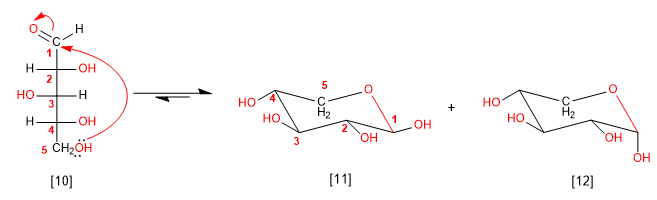
D-Arabinose
b -D-Arabinopyranose
a -D-Arabinopyranose