Mutarotation is the interconversion between anomers via the open form. Thus, glucose is found in the aqueous medium as a mixture of alpha and beta anomers and a small amount in open form. Let's look at that balance.
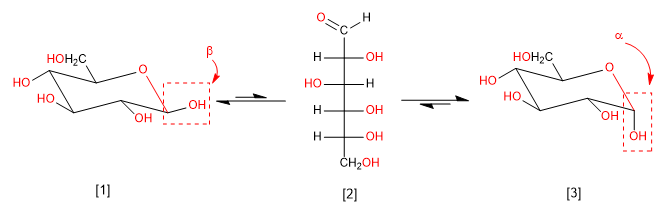
b -D-Glucopyranose (63.6%)
D-Glucose (0.003%)
a -D-Glucopyranose (36.4%)
This equilibrium with the open form allows the passage from the alpha to the beta anomer and vice versa. Once equilibrium is reached, a constant and characteristic composition of each sugar is established for the three species present in the medium. In the case of glucose, the predominant anomer is beta due to its greater stability (all equatorial hydroxyls).
Before going on to see the reactions of sugars, we are going to cyclize fructose, drawing the hemiacetals in a Haworth projection.
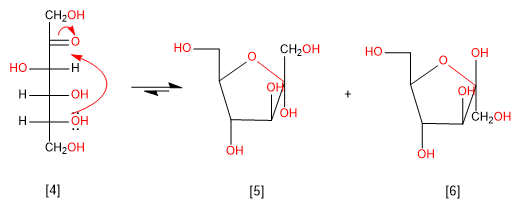
D-Fructose
a -D-Fructofuranose
b -D-Fructofuranose
In the case of fructose, the pyranose form is more stable than the furanose