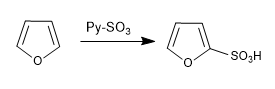
a) Sulfonation of furan:
The furan sulfones with the SO 3 -Py complex
d) Vilsmeier formulation
Furan reacts with dimethylformamide and phosphorus oxytrichloride, followed by basic hydrolysis, to form furan-2-carbaldehyde.
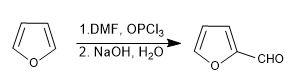
The mechanism is analogous to that described for pyrrole.
e) Mannich reaction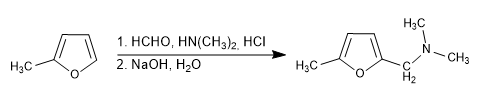
Given the lower reactivity of furan compared to pyrrole and thiophene, the presence of activators on the ring is convenient for this reaction to proceed with good yield.
The Mannich reaction employs methanal, a primary or secondary amine, and hydrochloric acid as reagents. A basic medium stage is used to neutralize.

f) Acetylation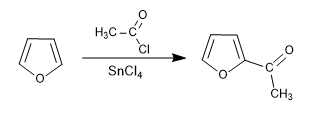
Furan is acetylated in the presence of acid halides or anhydrides with acid catalysis (SnCl 4 )
