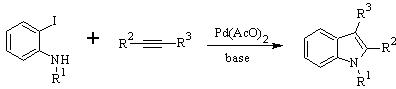Synthesis of INDOLES
(By the method of disconnections)
The indole ring system has been found in many natural compounds of great chemical and biochemical interest, which is why it is said to be the most abundant in nature. Thus, tryptophan is an essential amino acid, indigo is a dye, and indolyl-3-acetic acid is a plant growth hormone. On the other hand, interest in these molecules arises from their pharmacological use, examples being sumatriptan (antimigraine) and frovatriptan, also an antimigraine.
Indole is a colorless crystalline solid with a mp 52°C, easily soluble in most organic solvents and crystallizes from water, has a pleasant odor and is therefore also used as a perfume base.
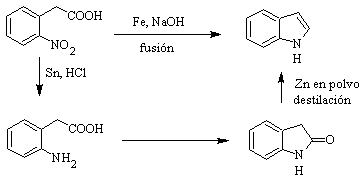
It was first prepared in 1866 by heating oxindole with zinc dust and has become an important commercial product. Baeyer in 1869 proposed the following synthesis:
The classical synthesis methods for indoles are those of Fischer, Bischler, Reissert and Leimgruber-Batcho, Bartoli, Larock, Gassman, Sugasawa, Fukuyama, Hegedus, and Dobbs.
1.
FISCHER synthesis
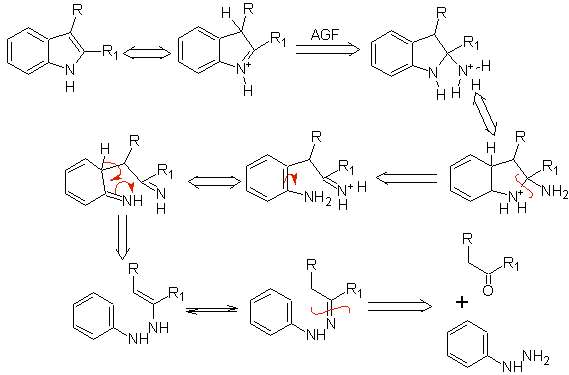
It consists of heating phenylhydrazones of ketones or aldehydes, with anhydrous zinc chloride, boron trifluoride, polyphosphoric acid, or some other acid catalyst, to produce indoles. An acid-catalyzed rearrangement of a phenylhydrazone occurs with elimination of water and NH 3 . Electrodonor groups favor cyclization and electroattractors hinder it.
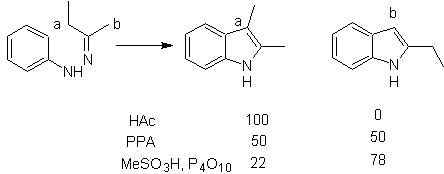
With asymmetric ketones, the intramolecular cyclization of the hydrazone can lead to two isomeric indoles in different proportions depending on the conditions used; in strongly acidic media, the less substituted indole can predominate.
When there are meta substituents, with respect to the hydrazone nitrogen, cyclization can take place in two positions, leading to two isomeric indoles:
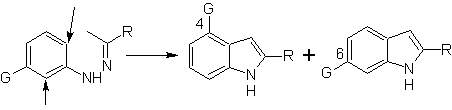
If the substituent G is electro-withdrawing, the two isomers (4- and 6-) are formed in approximately the same proportion. On the other hand, if G is an electron-donating substituent, the 6-substituted isomer is formed mainly. The retrosynthetic analysis of the indole formed by the Fischer synthesis can be considered as follows:
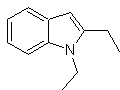
Propose a synthesis plan for the following molecules: | MOb 119
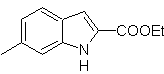
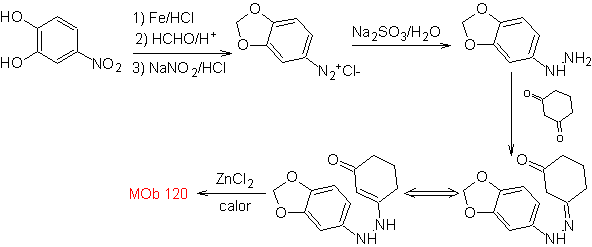
| MOb 120
|
MOb 119. Retrosynthetic analysis. The fundamental disconnection in the indoles that are supposed to be formed by the Fischer synthesis corresponds to a retro-transposition, which is shown in the disconnection of
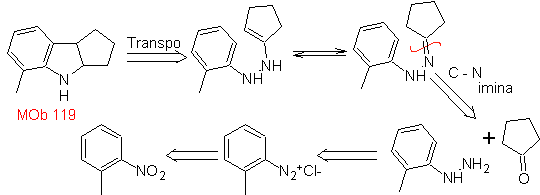
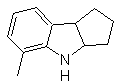
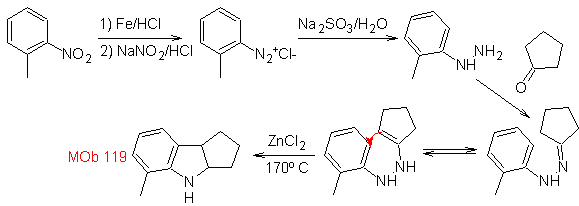
Synthesis : From ortho-nitrotoluene, the intermediate derivative of phenylhydrazine is generated, necessary in the synthesis of Fischer indoles, the imine is formed with a cyclopentanone, and by heating
MOb 120. Retrosynthetic analysis. The retro-transposition of
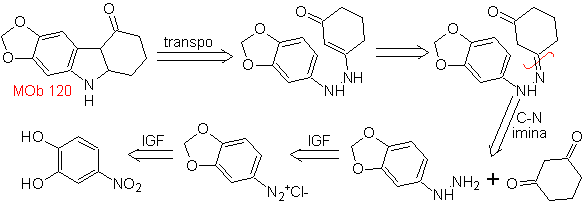
Synthesis. The OH of the starting molecule is protected, forming a cyclic acetal and the nitro group is reduced to later diazotize the amino.
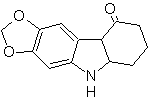
The reduction of the diazo compound formed with sodium sulfite, allows obtaining the phenylfidrazine derivative, which is combined with cyclohexanone, which then leads to
1.
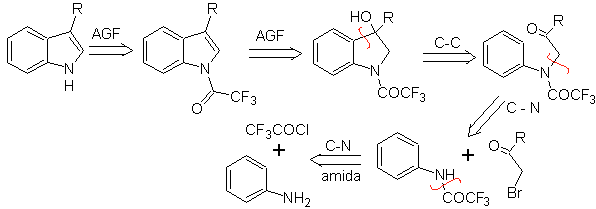
BISCHLER synthesis
It consists of an acid-catalyzed cyclization of an α-arylaminoketone, which is prepared from an aniline and an α-halocarbonyl. Using N-acylated α-aminoketones, the cyclization is more controllable and allows obtaining substituted indoles in the heterocyclic ring
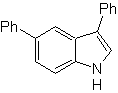
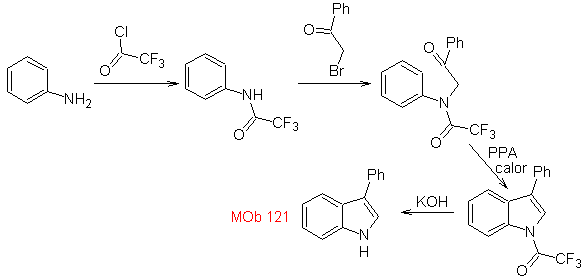
Propose a synthesis design for the following molecules : | MOb 121
| MOb 122
|
MOb 121 . Retrosynthetic analysis.
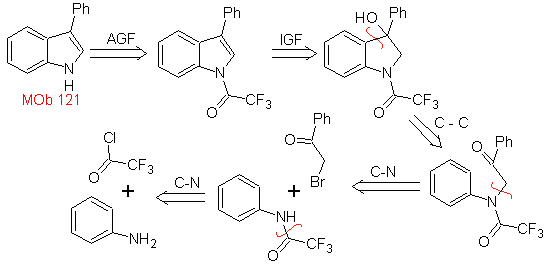
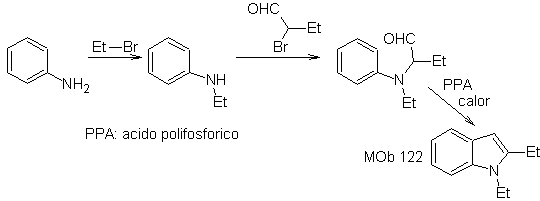
Synthesis. Starting from aniline, the required amide can be obtained, which will then react with α-bromo benzophenone, to form a molecule that cyclizes with PPA. The application of a base such as KOH and heat, forms
MOb 122 . Retrosynthetic analysis.
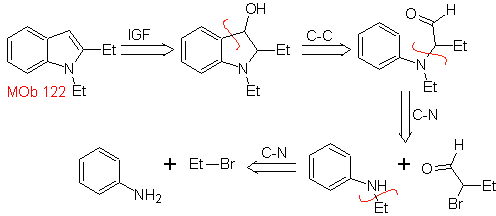
Synthesis. Again, the synthesis of
In this method it is essential that the hydrogens of the substituent in the ortho position to the nitro group are acidic enough, and therefore the nucleophile is guaranteed in its formation, to combine with a carbonyl compound.
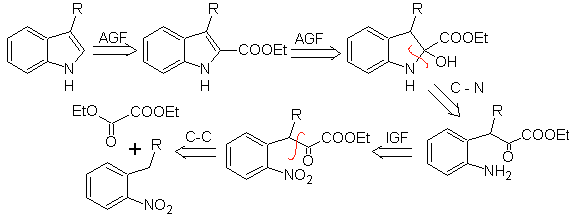
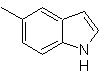
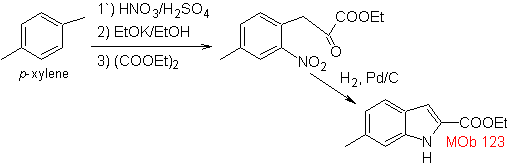
Propose a synthesis plan for the following molecules: | MOb 123
| MOb 124
|
MOb 123 . Retrosynthetic analysis.
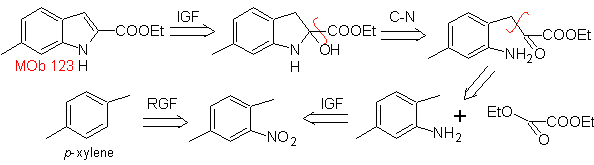
Synthesis. p-xylene is transformed into a nitroderivative, as the required intermediate, to cyclize, decarboxylate and thus form
MOb 124 . Retrosynthetic analysis.
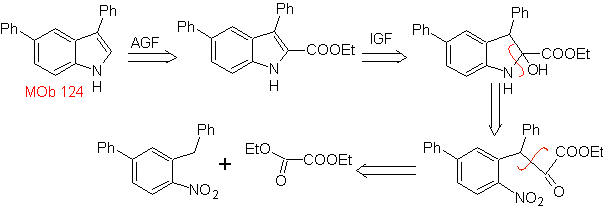
Synthesis. The starting material proposed for the synthesis of
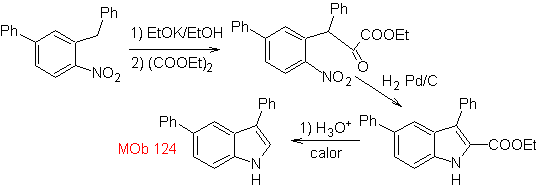
3.
LEIMGRUBER-BATCHO synthesis
As in the previous method, the acidity of the substituent in the ortho position to the nitro group must be guaranteed, the electrophile that is required is provided by the aminodiacetal.
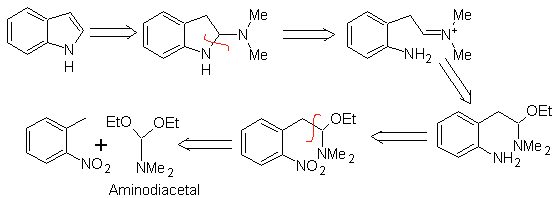
Propose a synthesis plan for the following molecules: | MOb 125
|
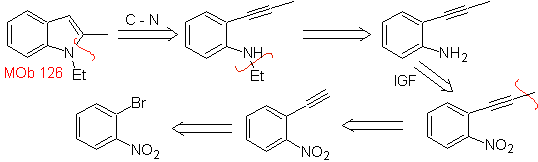
| MOb 126
|
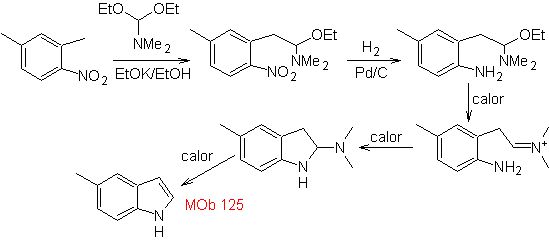
MOb 125. Retrosynthetic analysis. The disconnection strategy that emerges from the Leimgruber-Batcho synthesis is used to
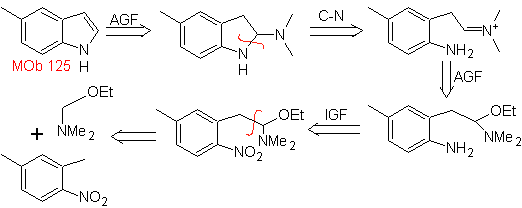
Synthesis. The intermediate 2,4-dimethyl-1-nitrobenzene can be prepared from benzene and continue with the reactions provided for in the Leimgruber-Batcho method, for the synthesis of
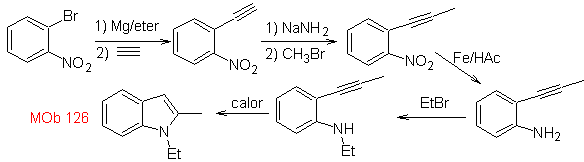
MOb 126 . Retrosynthetic analysis. The methyl group at C2 of the indole forces the disconnections to be linked to the presence of an acetylenic group, which will eventually combine with the amino group.
Synthesis. To introduce the acetylene group into benzene, an organomagnesium is reacted with acetylene. The cyclization is produced by a reaction of the amino group with the triple bond. The following reactions make it possible to form
HEGEDUS synthesis :
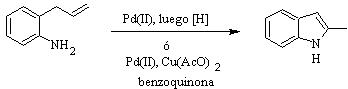
FUKUYAMA synthesis :
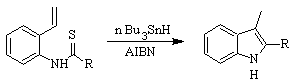
SUGASAWA synthesis :
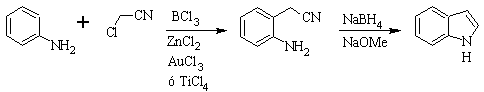
Gassman's synthesis :
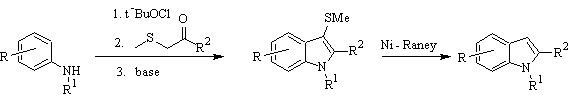
BARTOLI synthesis:
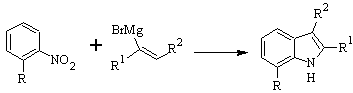
DOBBS synthesis :
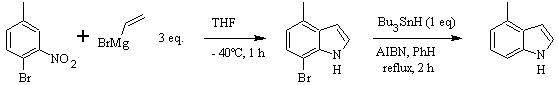
CASTRO synthesis:
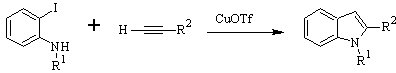
LAROCK Synthesis: