Isoquinoline synthesis
(By the method of disconnections)
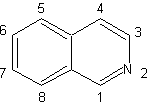
| Isoquinolines differ structurally from quinolines in the position nitrogen, since the latter is not fused, so it presents an "aliphatic reactivity". It is not found free in nature, but the isoquinoline cycle is found in some alkaloids, in aromatic or reduced form, eg papaverine. |
The best known synthetic methods for the preparation of isoquinolines start with 2-phenylethylamines and involve a cyclization through an additional carbon provided by the carbonyl group of another compound.
The main synthetic methods are: the Pomeranz-Fritsch synthesis, the Bischler-Napieralski synthesis, the Pictet-Gams synthesis and the Pictet-Spengler synthesis.
1. Synthesis of POMERANZ-FRITSCH.
This method of synthesis of isoquinoline occurs in two stages:
to.
First, benzaldehyde (1,3-electrophile-nucleophile) is condensed with aminoacetaldehyde diethylacetal (1,3-nucleophile-electrophile) to form a stable aldimine.
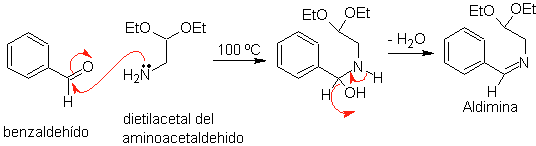
b.
Subsequently, the aldimine cyclizes in a strong acid medium, to an imine, with simultaneous elimination of ethanol, to produce an isoquinoline.
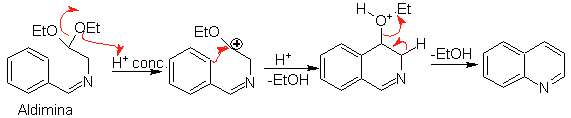
This second stage, being an electrophilic substitution, is subject to the effect that the electron-donating or accepting substituents have on the benzene ring in said reaction. However, due to the hydrolysis of the imine formed, in the strong acid medium used in the reaction, the yield of the process is reduced.
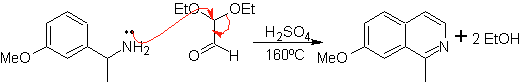
This method allows access to C-1 substituted isoquinolines, for which aromatic ketones have been tested, with very low yields. However, there has been greater success using the variant of appropriately substituted benzylamines as 1,4-dinucleophiles and glyoxal diethylacetal as 1,2-dielectrophiles.
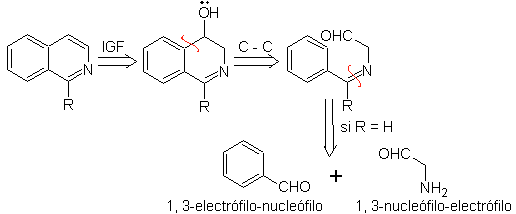
Something that must be made clear is that the Pomeranz-Fritsch method and its variant, previously analyzed, do not allow the preparation of isoquinolines substituted at C-3 and C-4 of the heteroatom. The retrosynthetic analysis of this method shows the possible intermediates involved in the reaction and the probable starting materials.
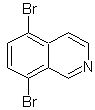
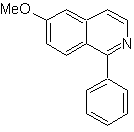
Propose a synthesis design for each of the following isoquinolines: | MOb 107
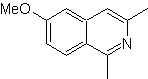
| MOb 108
|
MOb 107 . Retrosynthetic analysis . The disconnection of
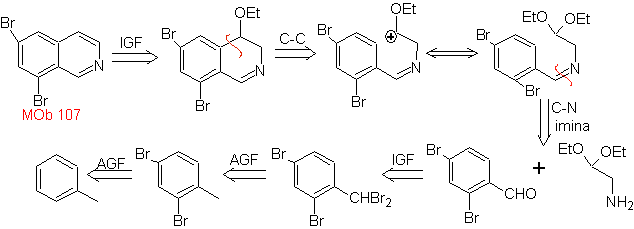
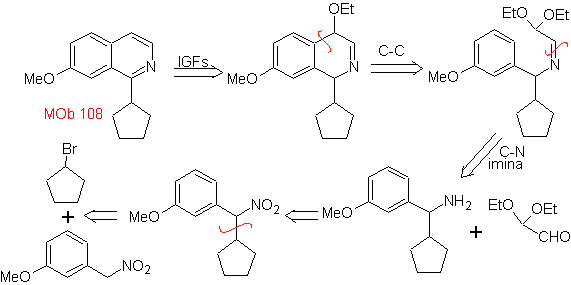
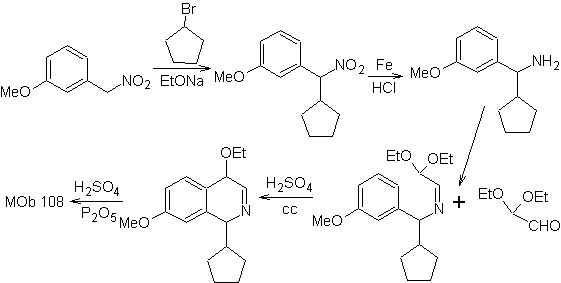
MOb 108. Retrosynthetic analysis . The presence of a substituent on C1 of isoquinoline, leads to disconnect
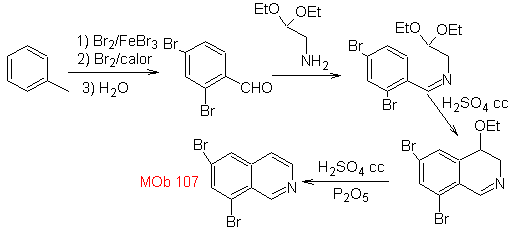
Synthesis The benzyalmine derivative is prepared to react with the aminoaldehyde diacetal, according to the Pomeranz-Fritsch synthesis, to form
2. BISCHLER-NAPIERALSKI synthesis .
This synthetic method of isoquinolines involves the reaction of a Phenethylamine (1,5-dinucleophile) with an acid chloride or anhydride (electrophile) to form an amide, whose cyclization with loss of water leads to a 2,4-dihydroisoquinoline with a substituent at C-1, which is oxidized to isoquinoline with Pd-C or phenyl disulfide.
The cyclization step is an electrophilic aromatic substitution and therefore will be favored by electron-donating substituents on the aromatic ring of phenethylamine. The m-substituted phenethylamines lead exclusively to C-6 substituted isoquinolines, due to cyclization in the para position with respect to the activating group.
The retrosynthetic analysis of the isoquinolines that are prepared by this method is as follows:
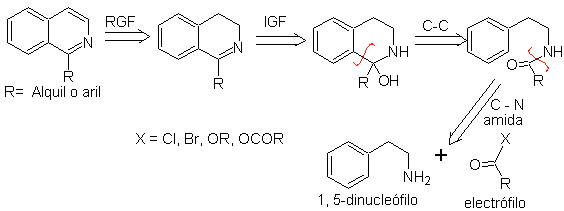
The cyclization agents most frequently used in this synthesis are:
to.
P 2 O 5 (phosphorus pentoxide)
b.
POCl 3 (phosphorus oxychloride) and
c.
SOCl 2 (thionyl chloride)
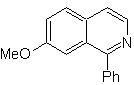
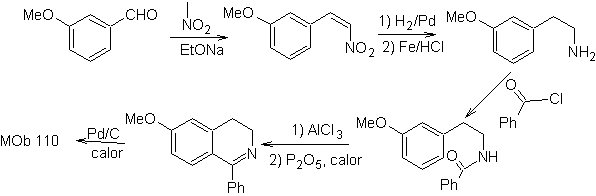
Propose a synthesis plan for the following isoquinolines: | MOb 109
| MOb 110
|
MOb 109 . Retrosynthetic analysis .
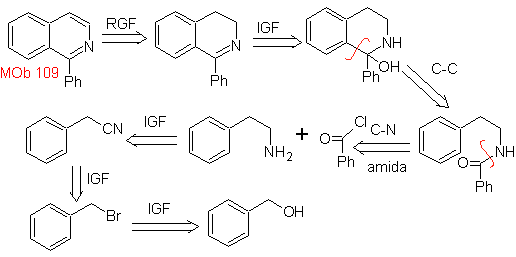
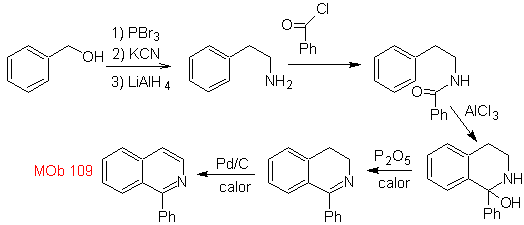
Synthesis. Benzyl alcohol is a good starting material to form phenethylamine, which is combined with benzoyl chloride. The product is cyclized and flavored with Pd/C and heat to form
MOb 110. Retrosynthetic analysis.
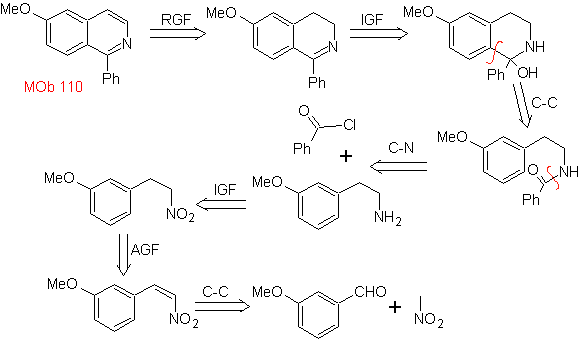
Synthesis. The synthesis of
3.
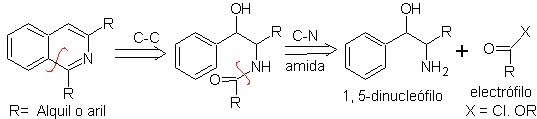
PICTET-GAMS synthesis.
It is a variant of the Bischler-Napieralski synthesis, in this method potentially unsaturated phenethylamines are used, obtaining a totally aromatic heterocycle, therefore the application of oxidants is not necessary.
Retrosynthetic analysis of this method shows the following potential reconnections and starting materials.
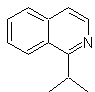
Propose a synthesis design for the following isoquinoline : | MOb 111.
|
MOb 111 . Retrosynthetic analysis . To initiate disconnection
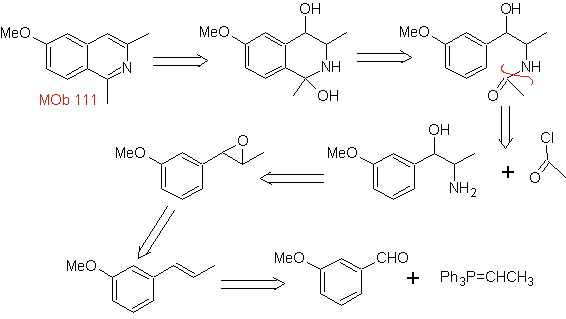
synthesis . The Pictet-Gams synthesis is applied, so it is not necessary to use an oxidant at the end, to reach the formation of
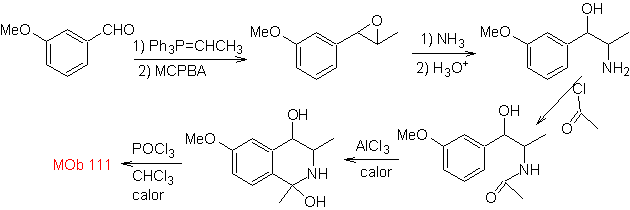
Phenethylamines can also react with aldehydes with good yields, giving aldimines that can cyclize in an acid medium to 1,2,3,4-tetrahydroisoquinolines, which must be oxidized to produce isoquinolines.
This cyclization requires suitably placed activating substituents to activate the positions ortho to the aminoethyl group, which is why the ring closure always occurs in the position para to the activator.
When the aromatic ring is activated with hydroxyl substituents, ring closure occurs under very mild conditions, due to the strongly activating effect of OH-
Retrosynthetic analysis of an isoquinoline formed by the Pictet-Spengler method , shows the following disconnections and starting materials:
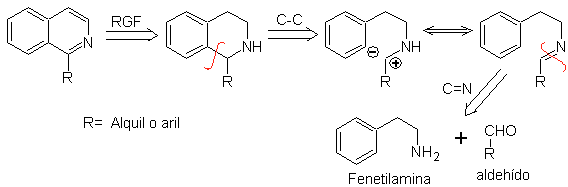
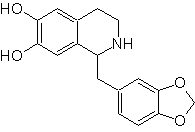
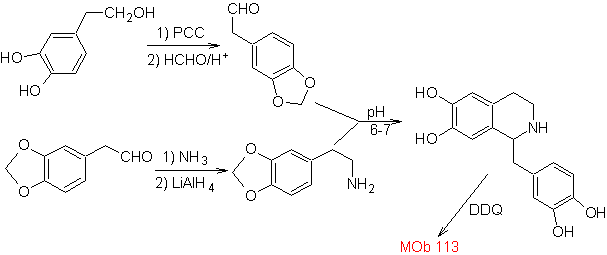
Propose a synthesis plan for the following Isoquinolines: | MOb 112
| ….. | MOb 113
|
MOb 112. Retrosynthetic analysis .
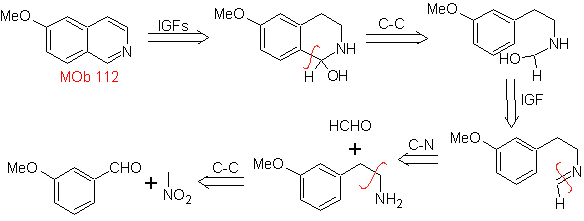
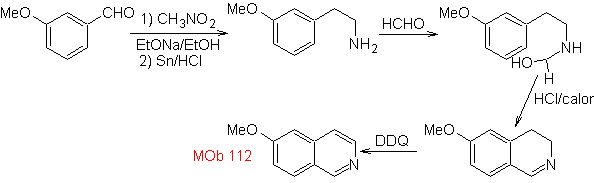
synthesis . The cyclization occurs in an acid medium and DDG is used to aromatize the hydroisoquinoline formed to prepare
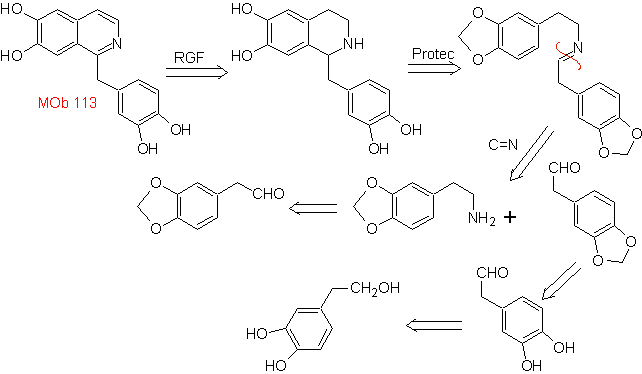
Synthesis The Pictet-Spengler synthesis is applied to form
Amé Pictet (1857-1937) was one of six members of the Swiss representation and acted as secretary of the Geneva Chemical Congress . As a representative of