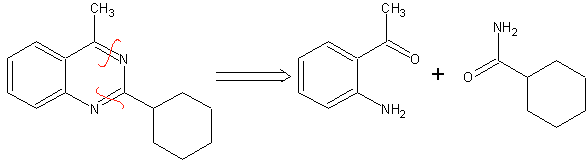
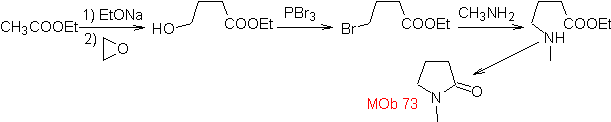SYNTHESIS OF HETEROCYCLES BY INTRAMOLECULAR CYCLATION
The construction of heterocyclic systems also uses these same reactions, with the particularity that the heterocyclic system must be present or contain at least one atom other than carbon. The most common are nitrogen, oxygen, sulfur and phosphorus.
The cyclic system of the molecule to be synthesized can come from the modification of a cyclic system present in one of the reagents involved in the synthesis or be the result of cyclization of non-cyclic antecedents and that has been built in the development of the synthesis by intramolecular cyclization or by methods based on intermolecular cyclizations (cycloadditions).
1. Intramolecular cyclization
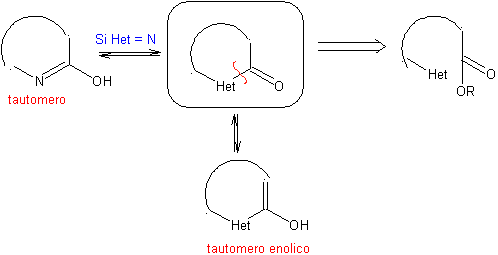
The general rules for the disconnection of heterocycles originating from an intramolecular cyclization, were adequately systematized by JI Borrell , the same as those assumed in this section (Het = N, O, S)
1.
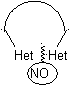
In the synthesis of a monocyclic compound, ring closure generally involves the formation of a carbon-heteroatom bond.
Model: |
|
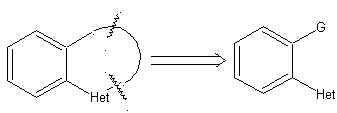
Example: |
|
|
|
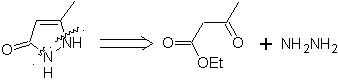
Example : |
|
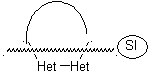
Model: |
|
Example: |
|
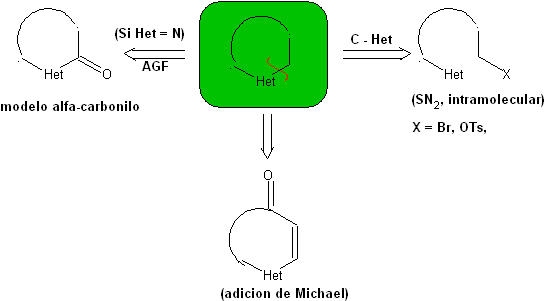
1.1. Models for intramolecular cyclization
Three models for intramolecular cyclization and the corresponding disconnections can be mentioned:
Saturated model:
| α-unsaturated model
| α-carbonyl model
|
1.1.1.
saturated model
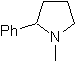
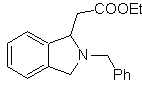
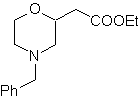
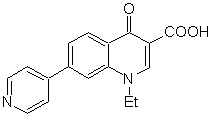
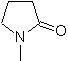
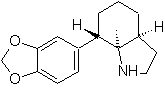
Propose a synthesis design, from simple and affordable materials, for the following molecules:
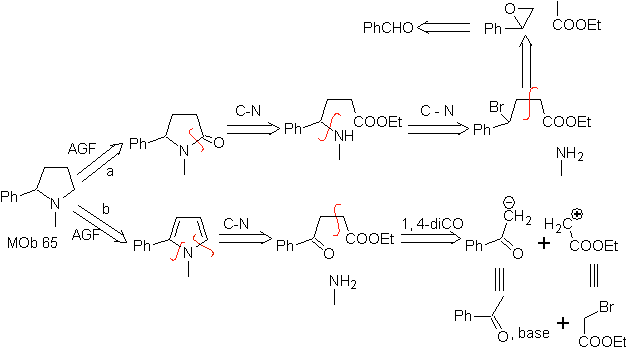
MOb 65
| … | MOb 66
| … | mob 67
|
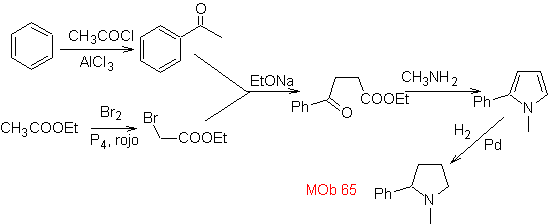
Synthesis. It begins with the Friedel-Crafts acylation of benzene on the one hand and on the other with the bromination of ethyl acetate, according to HVZ. The following stages require work in a basic medium and at the end we proceed to the hydrogenation of unsaturated centers to reach
MOb. 66. Retrosynthetic Analysis . The attack of the nucleophilic N on the β carbon, in relation to the ester group, (MOb 66) is orienting to think that it was formed by an intramolecular conjugated Michael addition of an amine on an α, β unsaturated ester. The C-N disconnection gives rise to other common disconnections, until reaching simple starting materials.
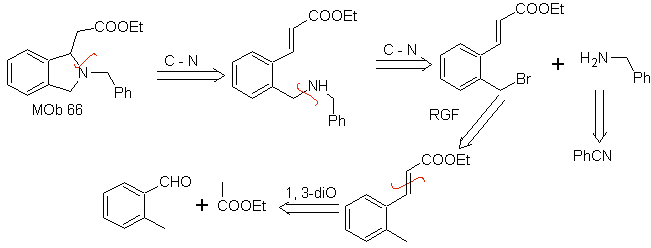
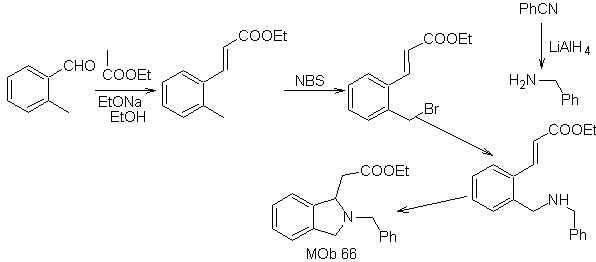
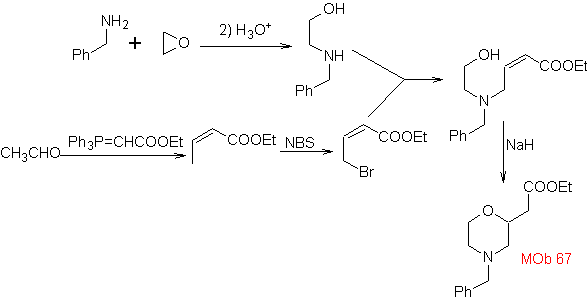
synthesis . The o-methyl benzaldehyde can be prepared, if necessary, by the Gattermann-Koch reaction on para-methyl sulfonic acid. The rest of reactions, for the synthesis of
MOb 67. Retrosynthetic analysis. As in the previous example, the CO bond at the β position to the ester group, in
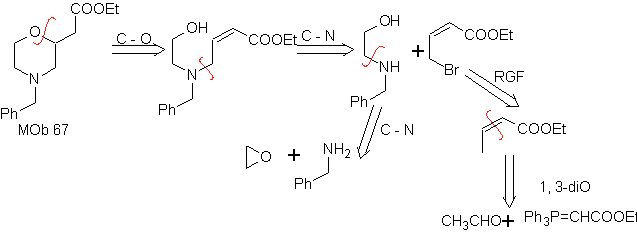
Synthesis. The synthesis of
1.1.2.
α-unsaturated model
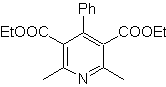
Propose a synthesis design from simple and affordable materials, for the following molecules:
MOb: 68
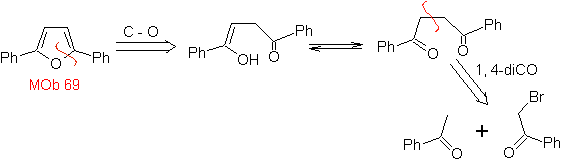
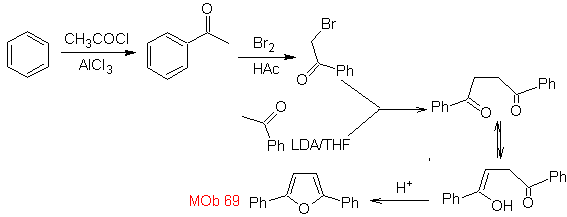
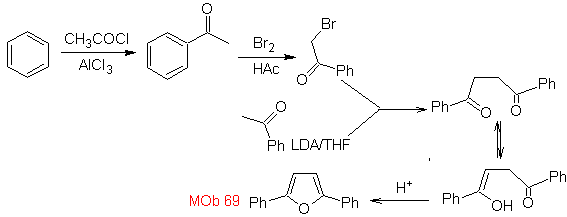
| MOb: 69
| MOb: 70
| MOb: 71
|
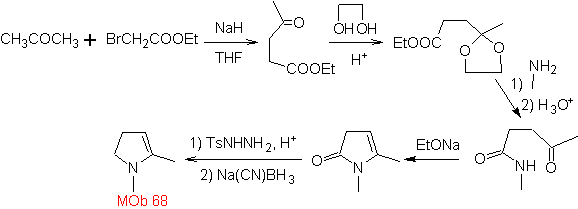
MOb 68. Retrosynthetic analysis . The disconnection process
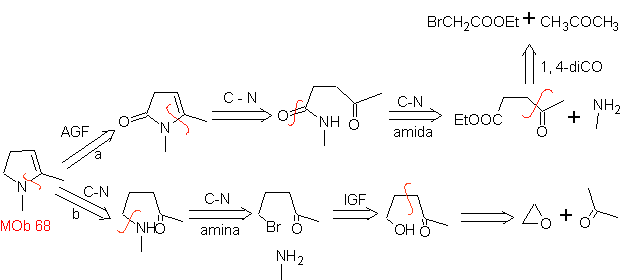
Synthesis. The ways of disconnection, of
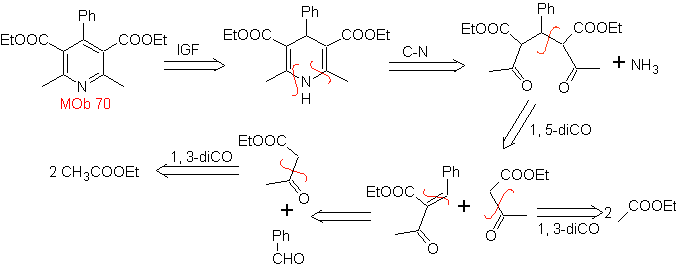
MOb. 70 . Retrosynthetic analysis . This MOb is a pyridine derivative, the structure it presents is characteristic of the products formed in the Hansch pyridine synthesis, that is, the pyridine cycle must be formed from an aldehyde and two moles of 1,3-diCO compound and oxidize the intermediate dihydroquinone formed .
MOb 71 . Retrosynthetic analysis.
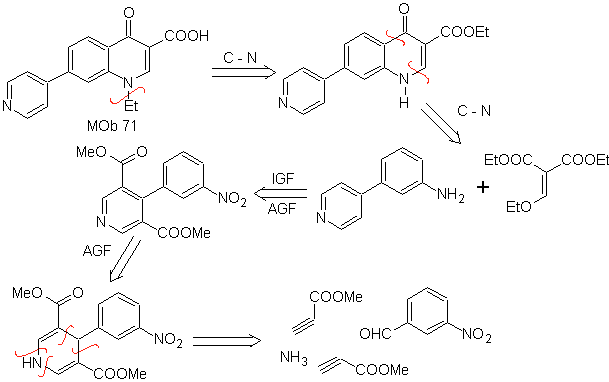
The simultaneous disconnection, on the ketone and the amine, shows the aniline and the carbonyl compound to have condensed. Since the introduction of the benzene ring into a pyridine is unlikely, the strategy of constructing the pyridine ring from appropriate acetylide derivatives is taken.
synthesis . Nitrile esters, together with m-nitrobenzaldehyde, allow intermolecular cyclization to form a hydropyridine derivative that is oxidized to pyridine with conc HNO 3 .
Then he nitro group, allows to build the quinoline pyridine ring, by condensation reactions with a suitable diCO compound. ethylation

Propose a synthesis plan, from simple materials, for the following molecules:
MOb: 72
| MOb: 73
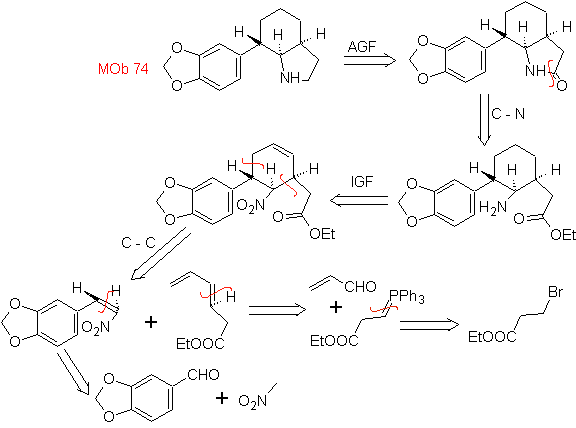
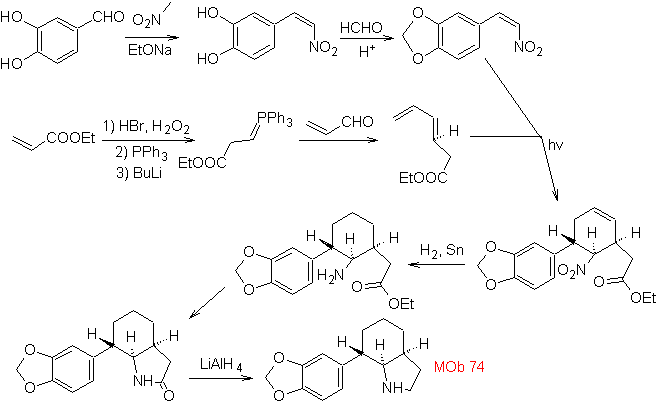
| MOb: 74
|
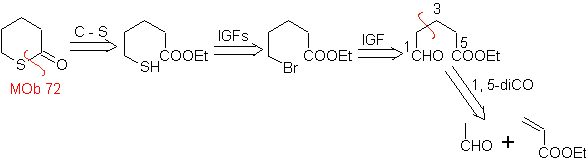
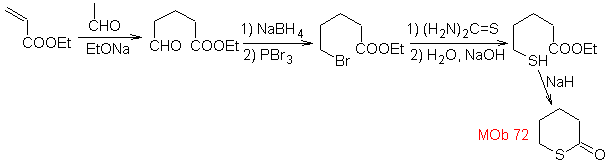
MOb 72. Retrosynthetic analysis . This MOb is a tetrahydropyranone, it is disconnected by the S-CO bond. The following IGFs allow the formation of a 1,5-diCO precursor, which, when disconnected, provides the starting materials.
MOb 73. Retrosynthetic analysis . Disconnection of the lactam amido bond generates the first precursor molecule, which is a γ. amino ester, which is formed between a primary amine and the γ-bromoester and consequently γ-hydroxyester, which is reacted between ethyl acetate enolate and an epoxide as simple and affordable starting materials.
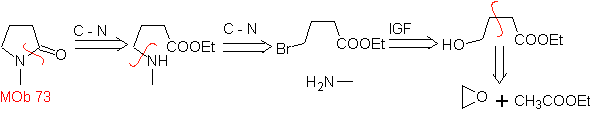
Synthesis : The epoxide and acetate ethyl are the reactants that react to form the γ -hydroester. The OH is replaced by bromine with PBr 3 and this reacts with methylamine. to form the precursor molecule that closes in a lactam ring, to form