Other synthesis strategies that use “illogical” syntons
1.
triple bond addition
The strategy of "adding" a triple bond, between two oxygenated functions in position 1,4, allows working later with a disconnection based on the chemistry of acetylides. In order to exemplify this strategy, let us see the elaboration of a synthesis plan for
MOb. 40. Retrosynthetic Analysis . A first IGF in
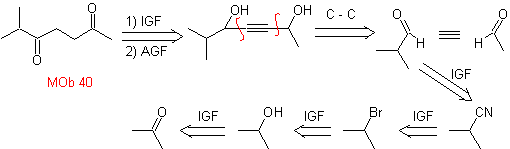
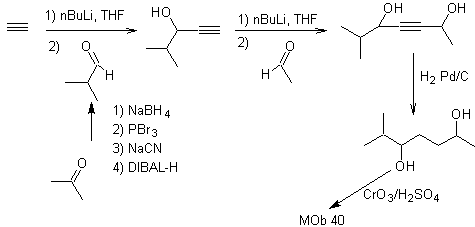
Synthesis. The diacetylide or acetylide in stages, combines with molecules of different aldehydes, the intermediate molecule formed is hydrogenated and then its alcohols are oxidized to the diketonic compound Mob 40.
The γ-lactones can also be prepared in an analogous way, as shown below:

2.
Adding the COOR group as an activating group
The addition of the COOR group, in addition to activating the anion synthon, facilitates the disconnection of a 1,4 diX molecule.
synthesize |
|
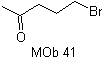
MOb 41. Retrosynthetic analysis.
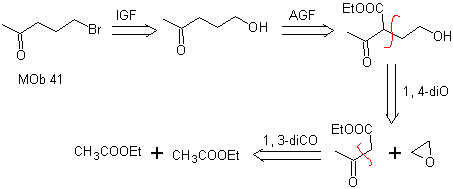
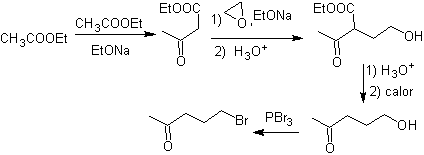
synthesis . Ethyl acetoacetate is a nucleophile that opens the epoxide in a basic medium.
The ester group is hydrolyzed and decarboxylated to arrive at
Propose a synthesis plan for the following molecules:
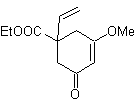
MOb 42
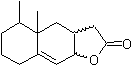
| MOb 43
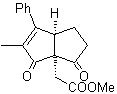
| MOb 44
|
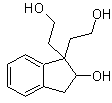
MOb 45
| MOb 46
| MOb 47
|
MOb 42 . Retrosynthetic analysis . The 1,3-diO disconnection of
Wacker oxidation: |
|
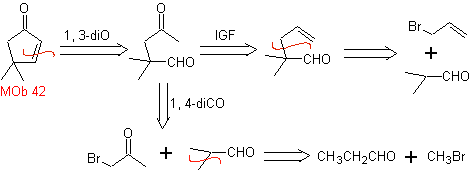
Synthesis. The allyl bromide alkylates the aldehyde, the product is oxidized and then cyclized in a basic medium, to form
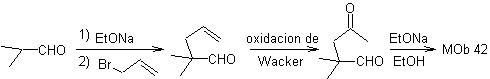
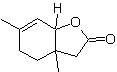
MOb 43, Retrosynthetic Analysis . The lactone, MOb 43, is opened, to originate a γ-hydroxy acid, which by IGFs, reaches a 1,4-ketoester, which is disconnected according to the model.
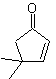
Cyclohexenone is formed by the Robinson annelation and 1,5-diCO is prepared from the Michael reaction.
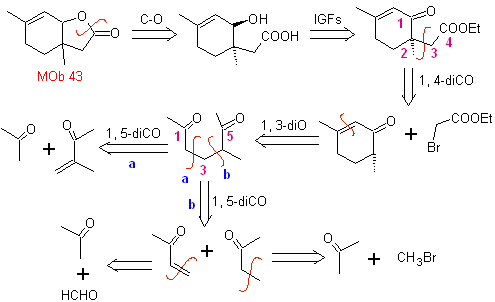
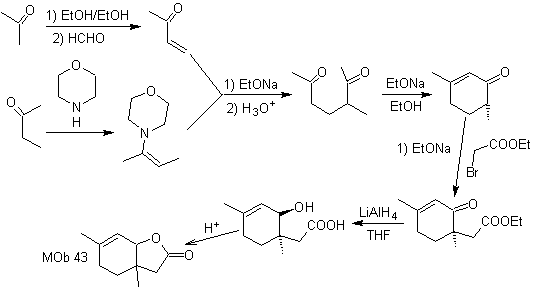
Synthesis. The secondary carbon enamine is obtained using a bulky secondary amine. The 1,5-diCO compound is cyclized by Robinson annelation and then reacted with the α bromo ester in a basic medium. The resulting diketone compound is reduced with LiAlH 4 and then, in an acidic medium, the lactone MOb 43 is formed.
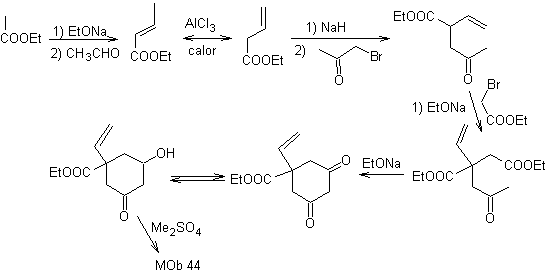
MOb 44 . Retrosynthetic analysis. The first IGF of
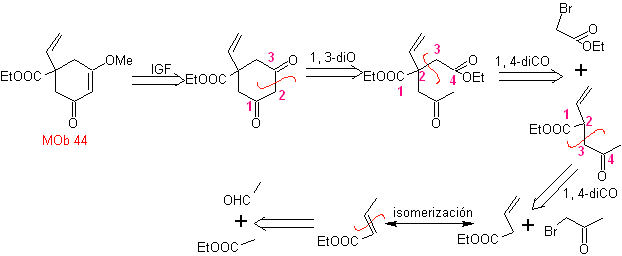
Synthesis. It is a sequence of condensations of the aldol or Claisen type. In order to synthesize
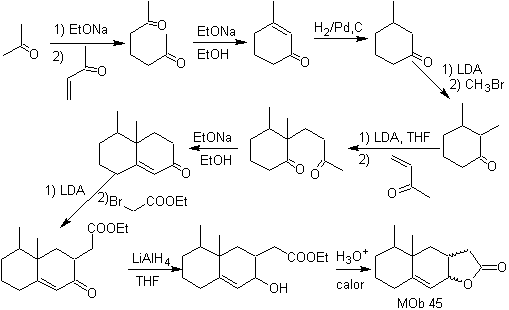
MOb 45 . Retrosynthetic analysis : The disconnection of the lactone function of
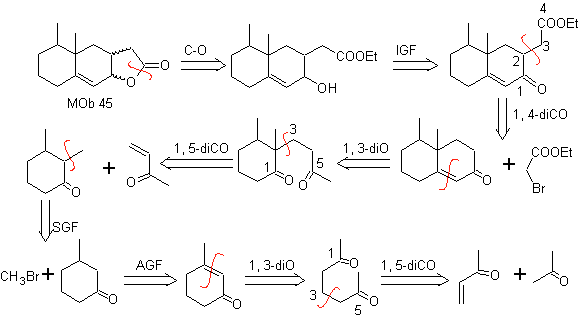
Synthesis: All the reactions used are linked to the Michael condensation, Robinson annelation and the Fischer ester formation reaction, it allows to obtain the lactone, that is to say
MOb 46. Retrosynthetic analysis. . It follows the 1,4-diCO disconnection, because it allows splitting into two parts
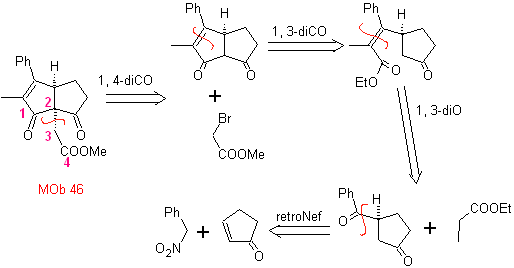
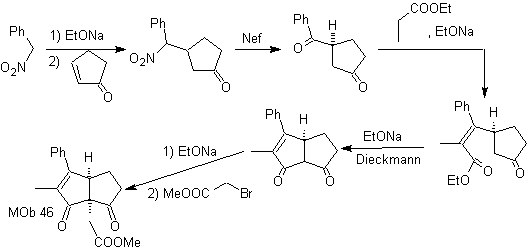
synthesis . According to Michael, the nitroarene nucleophile adds to α,β-unsaturated cyclopentanone. The nitro group is transformed into C=O, by the Nef reaction. The dicarbonyl compound is reacted with the ethyl propanoate enolate, followed by the Dieckmann reaction,
and the reactions necessary to arrive at
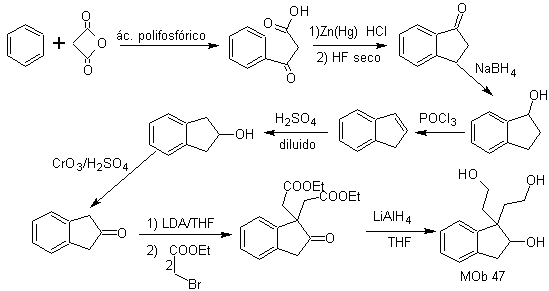
MOb 47. Retrosynthetic analysis.
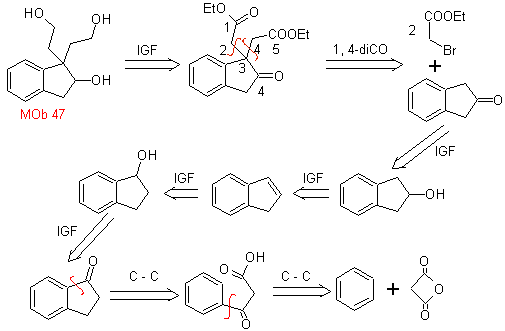
synthesis . The synthesis of