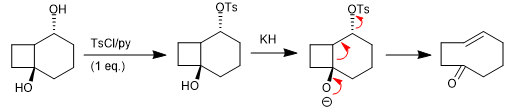
The Wharton fragmentation is a concerted reaction in which a leaving group is located in position 4 with respect to an electron donating group. The transfer of the free pairs of the donor group produces the fragmentation of the neighboring bond and the loss of the leaving group with the formation of double bonds. Let's see an example:
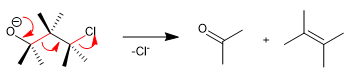
The reaction exhibits a definite stereochemistry involving the anti arrangement of the breaking bonds (drawn in red).
1,3-diols are frequent substrates in this reaction. They require prior conversion into monomesylates or monotosylates for later fragmentation in a basic medium.