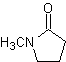
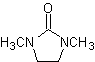
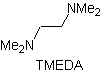
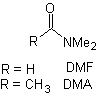Reactions of Enols and Enolates
Aldol reactions and the so-called condensation reactions of carbonyl compounds and others of this type, which can form enol and enolate structures, participate in a large group of important reactions that allow us to understand the existence of an immense number of molecules resulting from the interaction of enols or enolates with a series of electrophilic groups.
The study of this type of reaction has made it possible to verify and establish the existence of two reaction mechanisms through which they occur, as explained below:
TO)
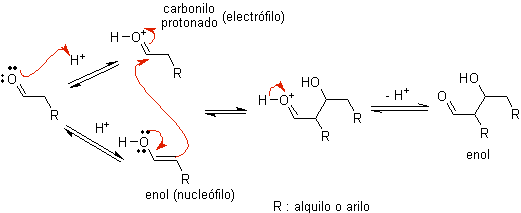
When acid is used as a catalyst, the carbonyl compound is initially protonated and then tautomerized to its enol form , which is a nucleophile on the alpha carbon to the carbonyl group. The same acid medium is enough to activate the carbonyl group of another molecule, making it highly electrophilic, which generates optimal conditions to produce an unsaturated carbonyl compound.
The reaction normally proceeds until the dehydration of the enol formed, catalyzed by the same acid of the reaction.
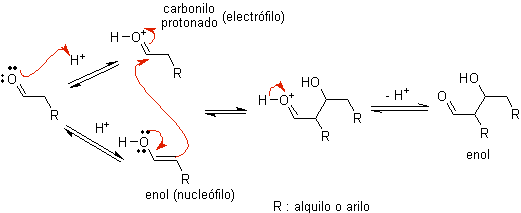
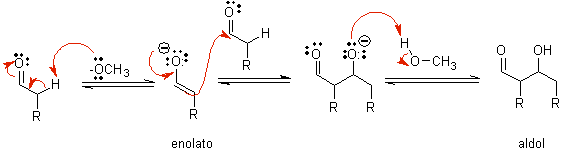
B) When the catalyst is a base, such as an alkoxide, the aldol-type reaction proceeds via the nucleophilic attack of the resonance-stabilized enolate on the carbonyl group of another molecule.
By dehydration of the aldol, catalyzed by base, the dehydrated final product is formed.
As in the previous case, the base-catalyzed dehydration (sometimes written as a single step), allows to control the reaction and produce a dehydrated final product. In some cases, the formation of enolates is irreversible.
how it looks only a catalytic amount of base is required in some cases, the most usual procedure is to use a estequiométrica amount of strong base such as LDA or NaHMDS . In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a later step.
Synthetic equivalents of various enols and enolates
enolate |
azaenolate |
nitroalkane enolate |
nitrile enolate |
| |||
ester, amide |
imine |
nitroalkane |
nitrile |
enol |
enol ether |
enol ester |
Silyl enol ether |
enamine |
Alkylation of enols and enolates:
The alkylation of enolates corresponds mostly to a nucleophilic substitution reaction with alkyl halides and epoxides. In this reaction, primary alkyl and benzyl halides are good for alkylation, secondary alkyl halides only in some cases, and tertiary alkyl halides basically do not react with enolates, because the reaction proceeds by a nucleophilic substitution mechanism. bimolecular
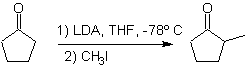
On the other hand, the speed of the alkylation is increased by the polarity of the solvents that are used as the reaction medium.
|
|
| ||
|
|
| ||
TORCH | NaHMDS | DIPEA | ||
Cy2BCl | Bu 2 BOTF |
Alkylation of silyl enol ethers , catalyzed by Lewis acids. Alkylation Y N 1


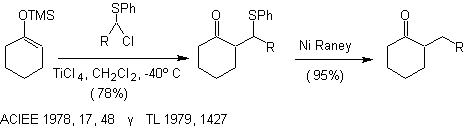
Alkylation of enolates of compounds a , b unsaturated carbonyls:
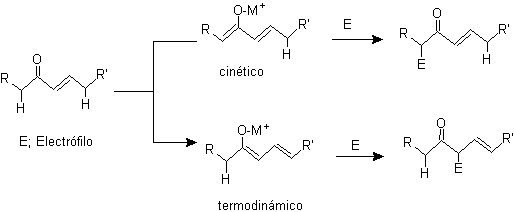
¨
g -alkylation of unsaturated ketones in a , b
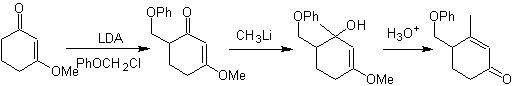
Alkylation of enamines .
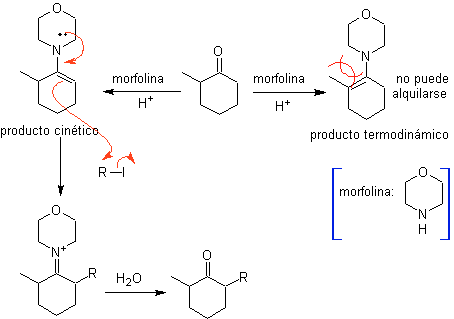
The monolaquilation and the formation of the kinetic product are controlled, taking advantage of the steric effect, for which a bulky secondary amine such as morpholine has to be used.
On the other hand, chiral enamines , produce in alkylation, also chiral alpha substituted ketones
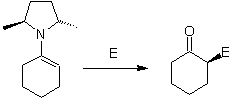
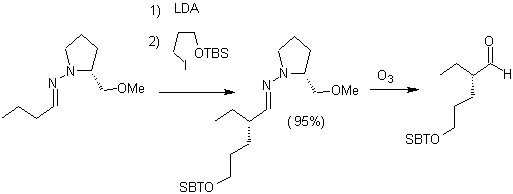
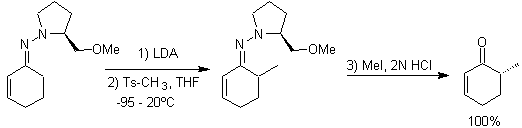
Alkyl imines: Imines, which are isoelectronic with ketones, can be transformed into enamines, which can then be alkylated or reacted with a reagent. electrophile.
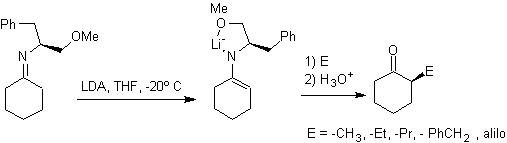
¨
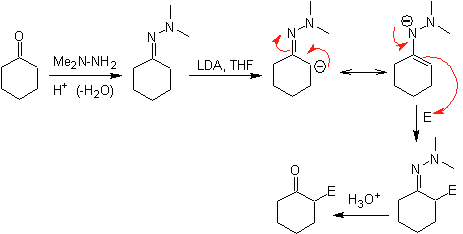
Hydrazone anions are much more reactive than the corresponding aldehyde or ketone enolates.
¨
There is a drawback, since there may be difficulty in the final hydrolysis.
¨
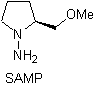
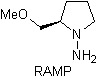
Chiral hydrazones are used for asymmetric alkylation (RAMP/SAMP hydrazones are used in asymmetric synthesis)
|
|