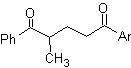
The 1,5-dioxygenated compounds are generally the result of conjugate addition reactions of nucleophiles from carbonyl compounds, with acidic H α (enols, enolates, enamines, etc.), as well as nitriles and nitrates, on substrates alpha beta unsaturated with respect to to carbonyl groups and the like, known as the Michael reaction, with complementary options being the Nef reaction and the Robinson annulation (annulation) reaction.
Disconnect model 1, 5 dioxygen (1,5-diO)
The 1,5-diO disconnection model can be applied, after the necessary functionalization, to compounds such as: 1,5-dihydroxyls, 1,5-hydroxyaldehydes, 1,5-hydroxyketones, 1,5-hydroxyesters, 1, 5-ketoaldehydes, 1,5-diketones, 1,5-ketoesters, 1,5-dialdehydes, etc.
The possibilities increase if nitroderivatives and nitriles are also taken into account, which can form very reactive carbanions in a basic medium capable of adding to α,β-unsaturated carbonyl compounds to obtain 1,5-diO type products.
The fundamental analysis of the disconnection of 1,5-diO compounds is as follows:
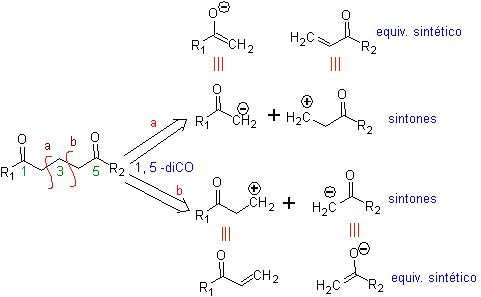
The choice of disconnection (a) or (b), around C3, will depend on the nature of the R1 and R2 groups, which may confer greater or lesser stability to the synthon or synthetic equivalent necessary for the formation of
Propose a synthesis design for MOb 29, 30 and
MOb 29
| MOb 30
| MOb 31
|
Solution:
MOb 29 . Retrosynthetic analysis: The carbanion needed to add to the a , b -unsaturated compound CO It can be obtained from diethyl malonate in a basic medium. Which will subsequently force a decarboxylation, to reach
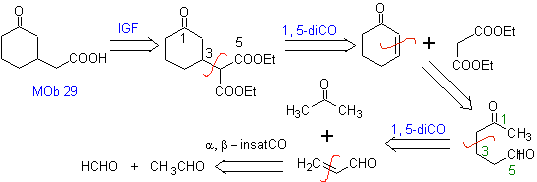
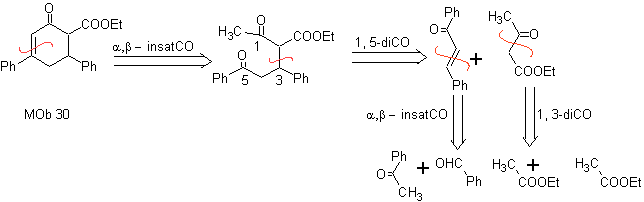
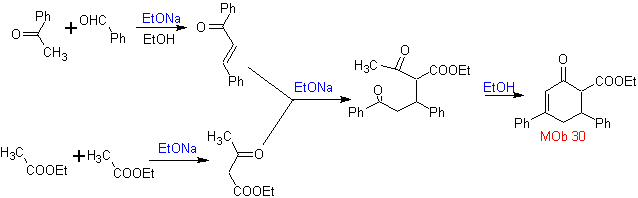
Synthesis. It proceeds with a combination of Claisen condensations, Claisen Schmidt , Michael's reaction and Robinson's annulment, to arrive at
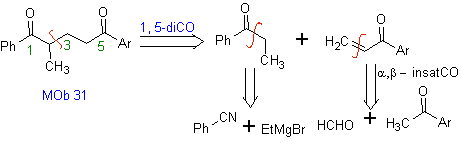
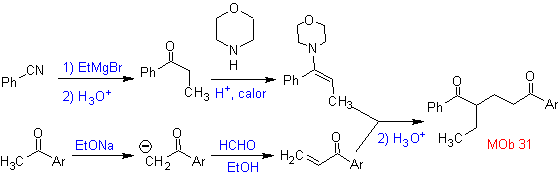
Vinyl ketones are very reactive and tend to dimerize by the Diels-Alder reaction, which is why, if they are necessary as a substrate in the Michael addition reaction, it is necessary to prepare them "in situ" and a very useful reaction for this is the Mannich reaction, as can be seen in the following example.
MOb 32 . Retrosynthetic Analysis. The initial application of the 1,5-diCO disconnection model generates a precursor such as vinyl ketone, which must be formed by the Mannich reaction, followed by Hoffmann elimination, to combine with the ketoester formed by the Claisen condensation.
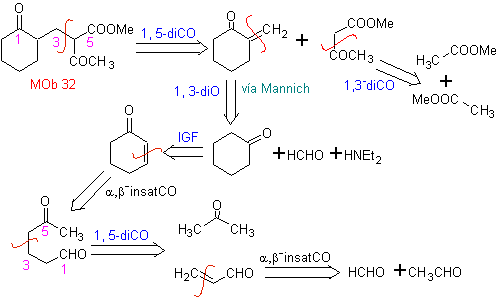
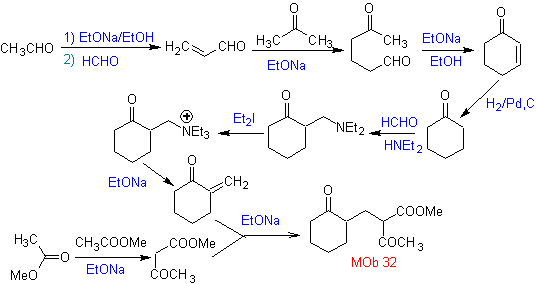
Synthesis. The vinyl ketone necessary for the (Michael) reaction with the ketoester is prepared by suitably combining the Mannich reaction and the Hoffmann elimination.
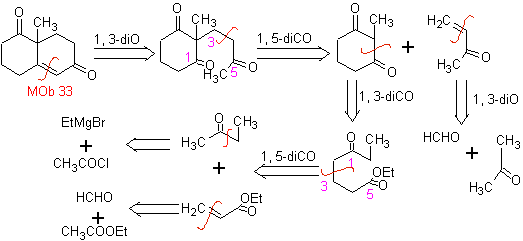
Synthesis. It will be necessary to exert control, to generate the nucleophile with the C 2º, which is achieved by the formation of the enamine, with a sufficiently voluminous amino group. The product is formed as indicated by the annulation or Robinson annulation.
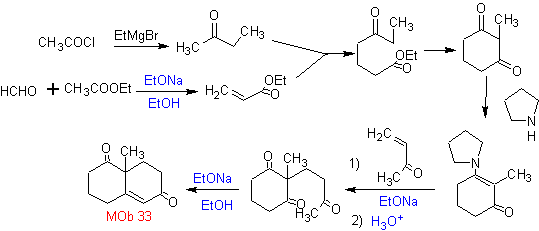
Robinson annelation also allows obtaining cyclic 1,3-diketone compounds.
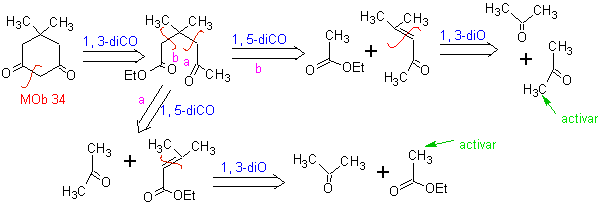
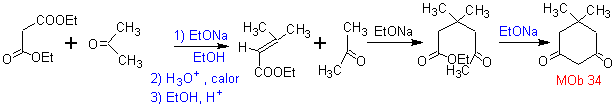
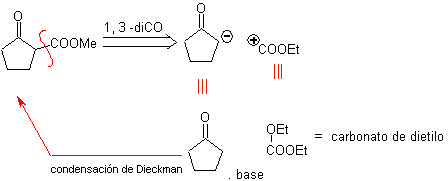
MOb 34. Retrosynthetic Analysis. The 1,3-diCO disconnection of