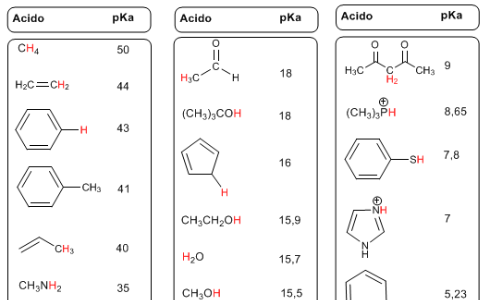
The pKa is defined as -logKa, and indicates the degree of acidity of the hydrogens of an organic compound.
A hydrogen is all the more acid the lower its pKa. The list begins with the compounds with the lowest acidity, the highest pKa, such as alkanes (pKa = 50).
The hydrogens with respect to esters, amides, carboxylic acids, aldehydes, ketones... present an intermediate acidity with pKa values between 20 and 30. The protonated species have hydrogens with negative pKas, reaching values below -10 .
The hydrogens with respect to esters, amides, carboxylic acids, aldehydes, ketones... present an intermediate acidity with pKa values between 20 and 30. The protonated species have hydrogens with negative pKas, reaching values below -10 .