Models-Alkanes
- Details
- Germán Fernández
- Models-Alkanes
- Hits: 165043
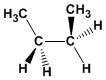
Formula : C 4 H 10
Molecular mass : 58,123
Melting point: -138.35ºC
Boiling point: -0.45ºC
Comment: The gauche conformation of butane alternates with methyls at 60º. After anti, it is the most stable conformation of butane.
- Details
- Germán Fernández
- Models-Alkanes
- Hits: 160870
Butane Molecular Model SIN
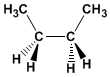
Formula: C 4 H 10
Molecular mass: 58,123
Melting point: -138.35ºC
Boiling point: -0.45ºC
Comment: Eclipsed conformation of ethane. The interaction between methyls makes this conformation the most unstable of butane.
- Details
- Germán Fernández
- Models-Alkanes
- Hits: 12146

Formula: CH 4
Molecular mass: 16.0426
Melting point: -182.47ºC
Boiling point: -161.45ºC
Comment: Highly flammable colorless gas. Lavatory.
- Details
- Germán Fernández
- Models-Alkanes
- Hits: 166816
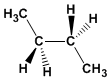
Formula: C 4 H 10
Molecular mass: 58,123
Melting point: -138.35ºC
Boiling point: -0.45ºC
Comment: It is the most stable conformation of butane. It presents the minimum interactions between substituents.
- Details
- Germán Fernández
- Models-Alkanes
- Hits: 14802
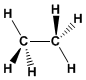
Formula: C 2 H 6
Molecular mass: 30.0694
Melting point: -183.3ºC
Boiling point: -88.6ºC
Comment: Colorless, flammable gas.