In this topic we will study six-membered heterocyles with two or more heteroatoms, focusing mainly on diazines (pyrazine, pyrimidine and pyridazine)
Diazines:
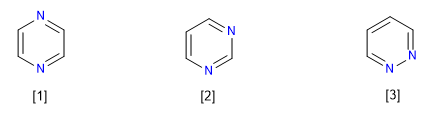
Pyridazine (1,4-diazine)
Pyrimidine (1,3-diazine)
Pyridazine (1,2-diazine)
Triazines:
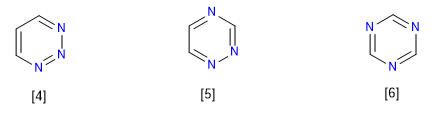
1,2,3-Triazine
1,2,4-Triazine
1,3,5-triazine
tetrazines:
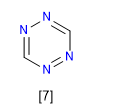
1,2,4,5-Tetrazine
The greater number of heteroatoms impairs the delocalization of the pi cloud, due to its electronegativity, lowering the aromaticity of these heterocycles in comparison with pyridine. In the case of pyridazine and 1,2,4-triazine, the NN bonds tend to be preferentially single, further increasing localization.
The greater number of heteroatoms favors nucleophilic addition and substitution reactions over electrophilic substitution reactions, which are practically non-existent.