This chapter addresses the fundamental aspects of the structure and stability of carbanions, focusing on those stabilized by carbonyl substituents and other electron-withdrawing groups (EWGs). A relationship is established between the properties and reactivity of these carbanions and their application as nucleophiles in synthesis. The acidity of C-H groups, determined by the stabilizing functional group, is fundamentally linked to the formation of enolates, highlighting the relationship between kinetic or thermodynamic control in enolate formation by deprotonation.
Control of the equilibrium between enolate and its conjugate acid is based on the choice of base. The reaction can be carried out under conditions where the enolate is in equilibrium with its conjugate acid or where the reactant is completely converted to its conjugate base. The amount and strength of the base are key determinants. Current procedures for enolate alkylation typically involve complete conversion to the conjugate base, allowing for greater regiochemical and stereochemical control. The solvent and other coordinating additives also significantly affect the structure and reactivity of carbanions generated by deprotonation.
The following table provides approximate pK data for various functional groups and some commonly used bases.
Compounds with two charge-stabilizing groups.
a-diketones, a-ketoesters, malonates, and other compounds with two stabilizing groups have pK values slightly below ethanol and other common alcohols. As a result, these compounds can be completely converted to enolates using sodium or potassium alkoxides. Often, the second EWG is extraneous to the overall purpose of the synthesis and its removal requires an additional step.
EWG: -NO2 > -COR > CN ~ CO2R > SO2R > Ph ~ SR > H > R
After 1960, procedures were developed that use aprotic solvents, especially THF, and amide-type bases such as lithium diisopropylamide (LDA). Dialkylamines have a pK around 35, which allows for the complete conversion of monofunctional compounds with pK > 20, especially ketones, esters, and amides, to their enolates.
[1] Lithium diisopropylamide (LDA)
Other commonly used bases are bis(trimethylsilyl)amide of lithium. The lithium, sodium, and potassium anions are abbreviated as LiHMDS, NaHMDS, and KHMDS. Disilammines have a pK around 30. The basicity of both dialkylamides and disilammines tends to increase with branching in the alkyl groups. More branched amides also show greater steric hindrance.
[2] bis(trimethylsilyl)amide of lithium or hexamethyldisilazide of lithium (LiHMDS)
LiHMDS is unstable and tends to form trimers.
Other bases, such as lithium tetramethylpiperidide, LiTMP, are sometimes used as bases for deprotonation. Other strong bases, such as the amide anion NH2-, the conjugate base of DMSO, and the triphenylmethyl anion, are capable of carrying out a virtually complete conversion of a ketone to its enolate. Sodium hydride and potassium hydride can also be used to prepare enolates from ketones, although the reactivity of the metal hydrides depends to some extent on the method of preparation and purification of the hydride.
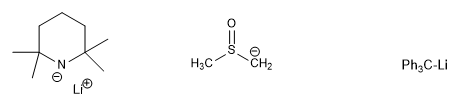
[1] LiTMP
[2] DMSO base
[3] Triphenylmethyl lithium