Synthesis of local anesthetics derived from benzoic acid
The properties of the alkaloids isolated from the leaves of the coca plant were discovered for the first time by Gaediche in 1855, the purification and isolation of the active principle called cocaine by Albert Nieman in 1860, practically began the history of local anesthetics. . Subsequently, Einhorn introduced procaine (novocaine) as a local anesthetic in medicine in 1904. |
|
Since then, humanity has witnessed a continuous and sustained development of the synthesis of new molecules with anesthetic active principles:
![]() In 1925 Niescher synthesized nupercaine.
In 1925 Niescher synthesized nupercaine.
![]() In 1928 Von Eisleb tetracaine (pantocaine) and
In 1928 Von Eisleb tetracaine (pantocaine) and
![]() In 1946 Lofgren and Lundquist synthesized lognicaine (xylocaine or lidocaine).
In 1946 Lofgren and Lundquist synthesized lognicaine (xylocaine or lidocaine).
![]() Then in 1954 Af Ekenstam and Egner obtained the synthesis of mepivacaine (scandicaine).
Then in 1954 Af Ekenstam and Egner obtained the synthesis of mepivacaine (scandicaine).
![]() Later in 1960 and 1964 they were introduced in
Later in 1960 and 1964 they were introduced in
![]() Finally, in the following years, new anesthetics have been incorporated into medicine.
Finally, in the following years, new anesthetics have been incorporated into medicine.
Local anesthetics are drugs that, when applied to a specific area of the body, produce a temporary and reversible loss of sensitivity (thermal, painful and tactile), without inhibition of the patient's consciousness. The duration of the effect of the drug depends on the dose used, its chemical structure, the formulation and the pharmaceutical form of the drug.
In general, local anesthetic drugs respond to different chemical structures, but all of them have similar effects or different intensities of the anesthetic effect. However, an attempt can be made to group them into benzoic acid esters, aminobenzoic acid esters, Amides, etc.
1 . Chemical structure of local anesthetics
Local anesthetics are predominantly weak bases and are formed by an arene group, ester or amide, which gives the molecule lipophilic properties (which mainly determine the potency of the drug), an aliphatic tertiary amino group (alkyl or alicyclic), which gives the molecule its hydrophilic character, and an alkyl intermediate chain that joins the parts of the arene with the amine and is responsible for the level of toxicity of the drug.
Thus, the main local anesthetics used in the different medical disciplines can be found in the following groups:
to)
Amino esters of benzoic acid :
b)
Esters of m-aminobenzoic acid :
c)
Esters of p-aminobenzoic acid :
d) Amides:
and)
Ketones :
F)
other groups
2 . Synthesis of local anesthetics derived from amino esters of benzoic acid
The most representative drugs of this group are cocaine, hexylcaine, piperocaine, ethyl aminobenzoate, meprilcaine, amylocaine, cyclomethicaine and propanocaine. These names respond to
Next, the synthesis of several of them will be presented in detail, as an example of the application of the synthesis methodology, known as the disconnection or synthon method. The order of exposure has no relation to the greater or lesser their importance, since the objective sought is to delve into the study of the chemical synthesis of these drugs. exist specialized treatises on the pharmacokinetics and pharmacodynamics of these molecules.
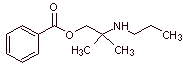
MOb 01: Hexylcaine hydrochloride, also called cyclaine (Merck) or osmocaine, is a short-acting local anesthetic. Propose a synthesis design, from simple and affordable materials, for this drug. |
|
Retrosynthetic analysis : The presence of the secondary amine in the structure of
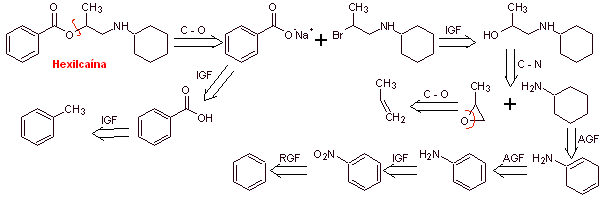
Synthesis of hexylcaine : The aminoalcohol required as an intermediate is prepared by the opening of the appropriate epoxide with the corresponding primary (nucleophilic) amine.
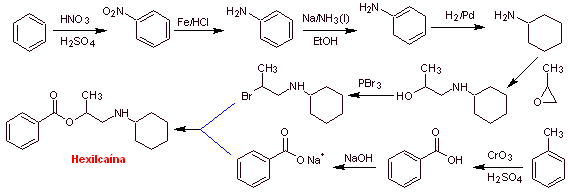
MOb 02. Piperocaine is a local anesthetic drug used as its hydrochloride salt. Propose a synthesis plan for this drug. |
|
Retrosynthetic analysis : The structure of
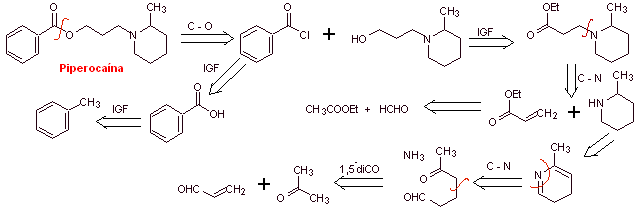
Synthesis of
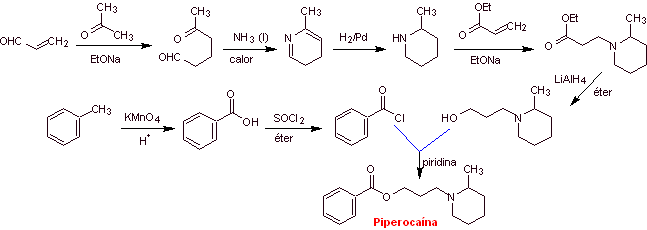
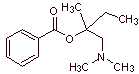
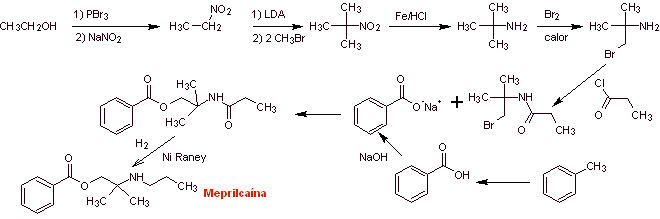
MOb 03: Meprilcaine, also known as epirocaine , is a local anesthetic of moderate duration. Propose a synthesis design for this molecule from simple and affordable materials. |
|
Retrosynthetic analysis : The structure of
The disconnection of the two precursor molecules is then continued, the CN bond of the amide is disconnected and this operation is continued until reaching ethyl acetate and toluene as starting materials.
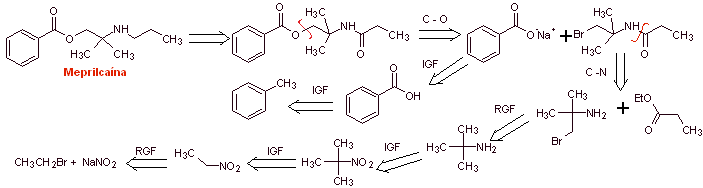
Synthesis of
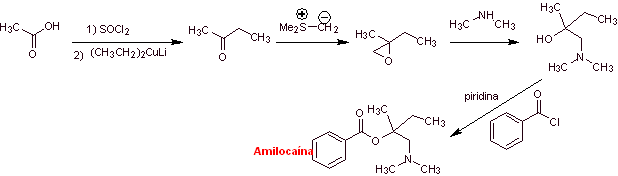
MOb 04: Amylocaine was the first synthetic local anesthetic patented by Ernest Fourneau Stovaine (French) at the Pasteur Institute in 1903. Propose a synthesis design for this molecule, from simple and affordable materials |
|
Retrosynthetic analysis : The disconnection process begins by the acyl-oxygen bond, the subsequent disconnection of the NC bond of the amino alcohol, discovers the structure of a substituted epoxide and dimethylamine as precursor molecules. The epoxide can be formed from a ketone with sulfur ylide.
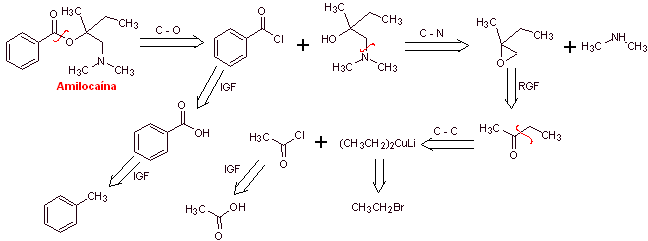
Amylocaine synthesis : The required 2-butanone precursor molecule can be prepared from acetic acid and the corresponding Gillman's reagent. Which leads to preparing the epoxide from the same ketone, using the respective sulfur ylide, to introduce the group methylene (–CH 2 -).
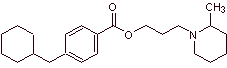
MOb 05 : Cyclomethicaine is another important local anesthetic. Propose a synthesis plan for this drug, starting from simple and affordable materials. |
|
Retrosynthetic analysis : The disconnection begins by the acyl-oxygen bond of the ester group and then continues with the disconnections of the two precursor molecules generated, until arriving at benzene, toluene, ketone and acetic acid as starting materials.
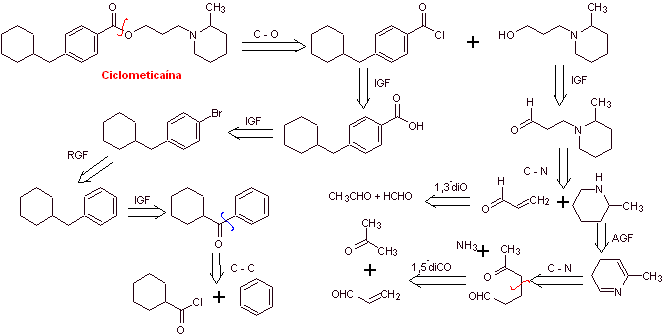
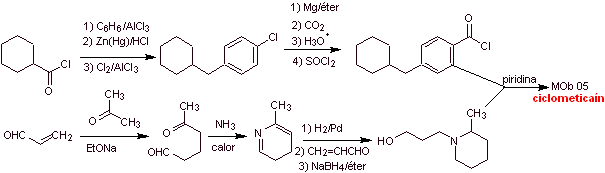
Cyclomethicaine synthesis :
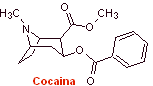
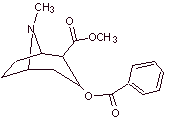
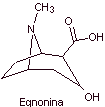
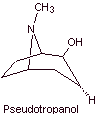
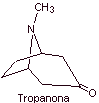
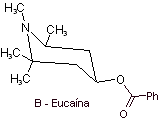
MOb 06 : Cocaine is the first local anesthetic of natural origin, obtained from coca leaves, and unfortunately it has been used mostly unscrupulously due to its hallucinogenic effects. Despite this, several cocaine derivatives have been obtained, all of them used as local anesthetics, such as ecgonine, |
|
| … |
|
|
|
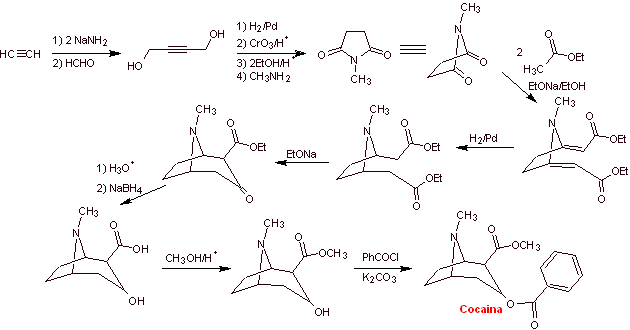
With essentially medical and therapeutic purposes, cocaine has been synthesized in chemistry laboratories. Describe a possible chemical synthesis route for cocaine from simple materials.
Retrosynthetic analysis : The disconnection is initiated by the acyl oxygen bond of the ester directly attached to the bicyclic compound, an aspect that greatly simplifies the structure and forms precursor molecules, much easier to deal with in their disconnections. Then egnonin is formed, the same one that is disconnected by a Claisen retro-condensation, to continue with a Knoevenagel retro. Subsequent disconnections originate precursor molecules that go as far as acetylene and formaldehyde as starting materials.
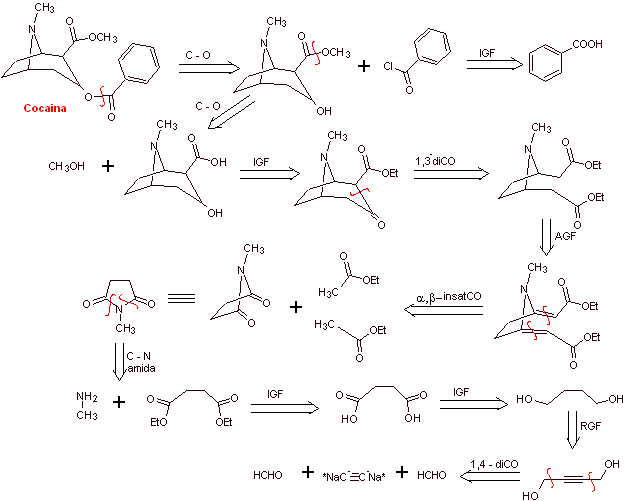
Synthesis of