SULFAMIDE SYNTHESIS
It is known that sulfonamides were the first antimicrobials to be used systemically. Its chemical structure is a benzene nucleus with amino groups that give it its activity. The amino group is acetylated in the liver, inactivating it. Depending on the substituent in said sector the drug is more active.
Given its similarity to para-aminobenzoic acid, it behaves as a competitive inhibitor of this substance, which is necessary together with dihydropteridine to synthesize dihydrofolic acid, an intermediate compound in the folate synthesis pathway.
Unlike more advanced organisms, bacteria need to synthesize their own folates [they do not acquire it from the environment], so sulfonamides, by inhibiting this process, inhibit the processes of synthesis of nucleic acids and are BACTERIOSTATIC.
TRIMETROPRIM
Trimethoprim is a derivative of 2,4 diaminopyrimidines such as | This compound INHIBITS the enzyme dihydrofolate reductase and prevents the formation of tetrahydrofolic acid, that is, they act in the same metabolic pathway as sulfonamides, but in a subsequent enzymatic reaction. Trimethoprim is never used alone, but when associated with sulfonamides they are potentiated in such a way that they become BACTERICIDAL, decrease the possibility of generating resistance and increase the antimicrobial spectrum. The association between sulfamethoxazole and trimethoprim is fixed: 1:5. For example, the commercial preparations Cotrimoxazole [forte or not] come with this reason. |
Sulfonamides, they are generally classified according to the duration of their action and the way the drug is applied, as well as other characteristics. Depending on the mode of action, sulfonamides can be:
to)
Short or intermediate acting sulfonamides.
to. General use sulfonamides
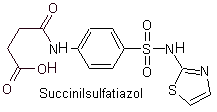
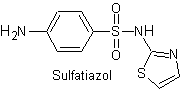
Yo. Sulfathiazole
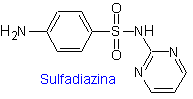
ii. sulfadiazine
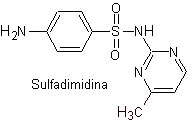
iii. Sulfadimidine
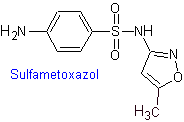
iv. Sulfamethoxazole (alone or associated with trimethoprim: cotrimoxazole)
b. Highly soluble compounds initially used in the treatment of urinary tract infections.
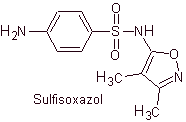
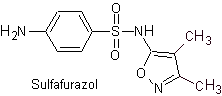
Yo. Sulfisoxazole
ii. sulfamethizole
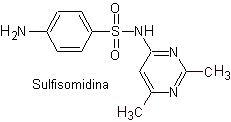
iii. Sulfasomidine
b)
Long-acting sulfonamides.
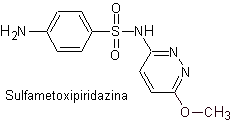
iv. sulfamethoxypyridazine
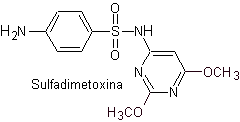
v. sulfadimethoxine
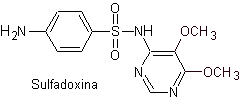
saw. Sulfadoxine
c)
Sulfonamides limited to the gastrointestinal tract
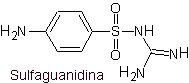
vii. Sulfaguanidine
viii. Sulfatalidine
ix. Sulfasuxidine
x. Sulfazolazine
d) Topical Sulfonamides.
xi. mafenide acetate
xii. silver sulfadiazine
xiii. sul facetamide sodium
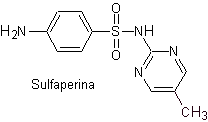
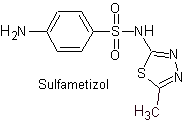
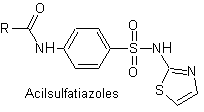
Main sulfas:
| ……… |
|
|
|
| …….. |
|
|
|
| …….. |
|
|
|
| …….. |
|
|
| |
|
|
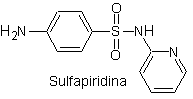
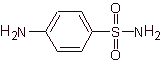
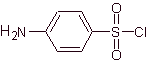
The structures that present the sulfas, generally exhibit two parts, one of them is the one corresponding to 4-aminobenzenesulfamide or 4-aminobenzenesulfonic chloride, depending on the reaction used to form the sulfa, and the other part is generally a heterocycle, which of course varies depending on the particular sulfa in question.
4-aminobenzenesulfamide | …. |
4-aminobenzenesulfonic chloride |
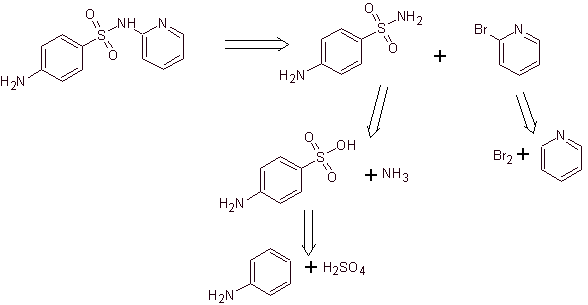
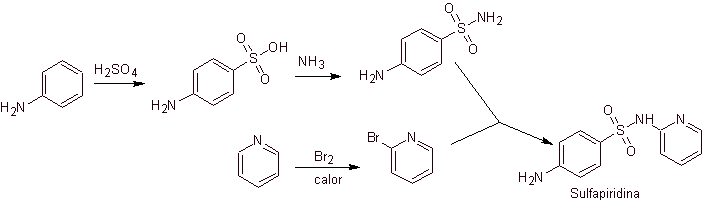
Synthesis of 4-aminobenzenesulfamide and
4-aminobenzenesulfonic chloride :
Retrosynthetic analysis:
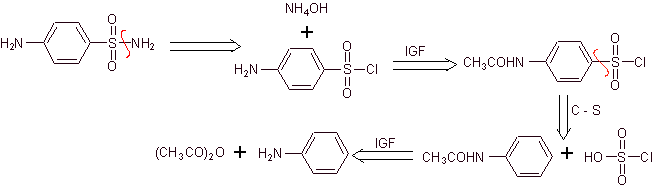
Synthesis:
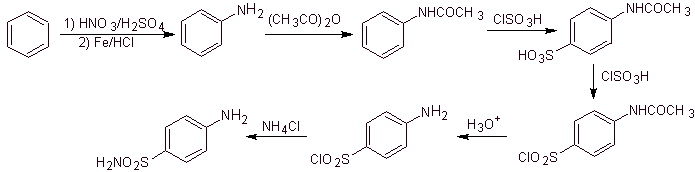
On the other hand, it is also possible to resort to any of the following reactions, to form the substrates (synthetic equivalents): that are necessary, to synthesize the various sulfonamides, as the case may be:
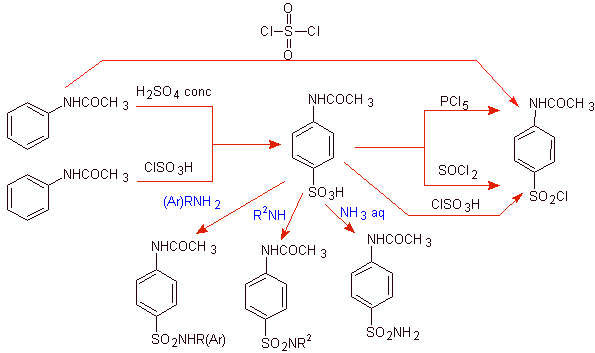
The aspects of strategy used recurrently are related to the following criteria:
![]() The amino group of the aniline is protected by acylation, to avoid the formation of the sulfonic amide, with the reagents used for the sulfonation of benzene: H 2 SO 4 , ClSO 3 H or SO 2 Cl 2 and
The amino group of the aniline is protected by acylation, to avoid the formation of the sulfonic amide, with the reagents used for the sulfonation of benzene: H 2 SO 4 , ClSO 3 H or SO 2 Cl 2 and
![]() The acetamido group of (acetanilide) can be hydrolyzed before reaction with the heterocycle to form the sulfa. Alternatively, proceed to stereoselectively controlled hydrolysis, at the end with dilute HCl, since the amide group of acetanilide is the first to hydrolyze.
The acetamido group of (acetanilide) can be hydrolyzed before reaction with the heterocycle to form the sulfa. Alternatively, proceed to stereoselectively controlled hydrolysis, at the end with dilute HCl, since the amide group of acetanilide is the first to hydrolyze.
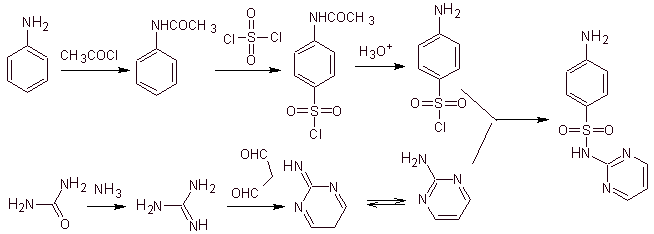
Example. Synthesize sulfadiazine (MOb 10), sulfathiazole (MOb 11) and sulfisoxazole (MOb 12), from simple and affordable materials.
MOb 10, Retrosynthetic analysis. Disconnection is initiated by the sulfonamide bond, resulting in two simple synthetic equivalents. One of them is 4-aminobenzenesulfonic chloride, the other is 2-aminopyrimidine, a heterocycle whose synthesis is not very difficult.
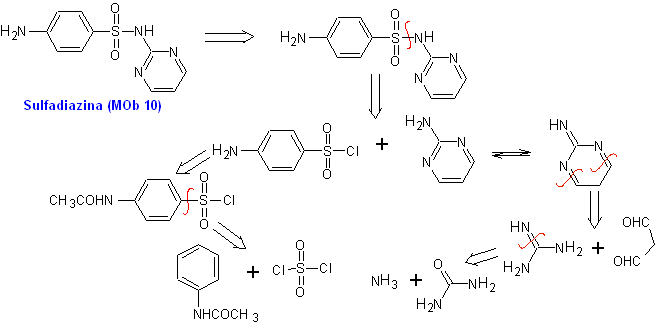
Synthesis of
MOb 11. Retrosynthetic analysis: Disconnection generates p-aminobenzenesulfonic chloride and the 2-amino-1,3,thiazole heterocycle.
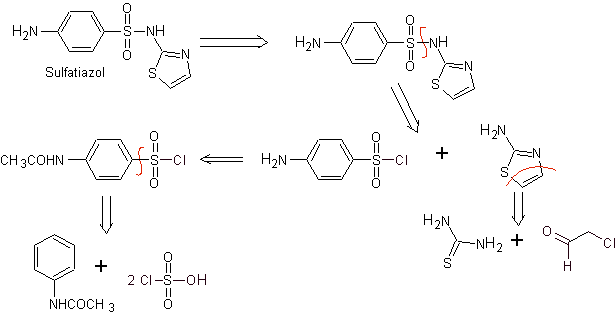
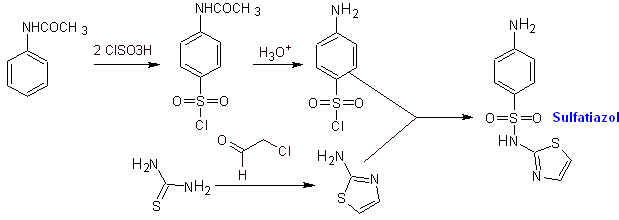
Synthesis of
MOb 12. Retrosynthetic analysis: disconnection gives rise to a new heterocycle, 5-amino-3,4-dimethyl-1,2-oxazole
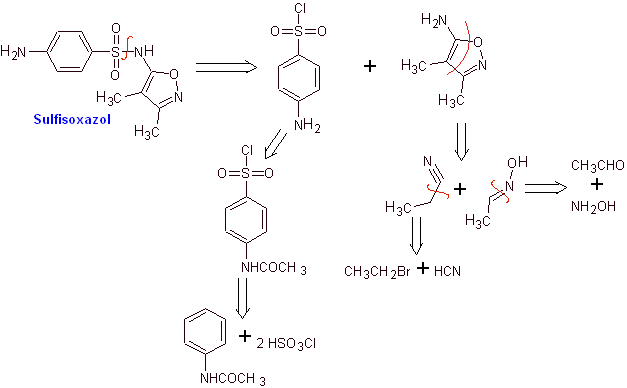
Synthesis of
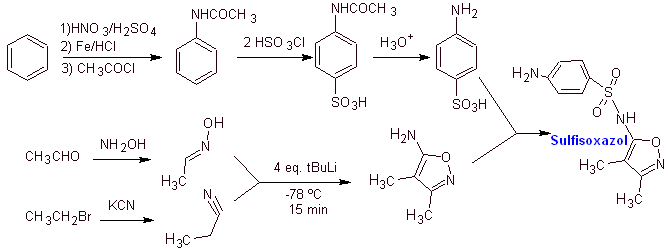
MOb 13. Synthesis of
retrosynthetic analysis