Control in organic synthesis is one of the most important tasks to achieve the required or planned transformation. and/or to avoid the formation of those by-products that substantially impair the optimal development of the organic synthesis in question. Likewise, from a more general perspective, the control can also include or cover the aspects of symmetry and selectivity.
Then, control should be understood as a series of synthetic operations that allow the chemist to form the carbon skeleton with the intended functionality or to "place" a group or atom in the required place or position.
Consequently, these operations may be of a varied range of routines with an intention reflected by the chemist and that demand certain cognitive abilities and skills similar to artistic ones, for the construction (synthesis) of organic molecules.
Therefore, in condensation reactions, as in others, control operations may be included in one of the following categories.
![]() Competing reactions (self-condensation and/or cross-condensation)
Competing reactions (self-condensation and/or cross-condensation)
![]() Activation – deactivation
Activation – deactivation
![]() Selectivity and specificity
Selectivity and specificity
![]() Protection-deprotection
Protection-deprotection
In the condensation reactions of carbonyl compounds, it is essential to establish the order of events in advance to minimize or, if necessary, suppress the possibilities of self-condensation and the occurrence of cross-condensation, which unfortunately are an evident threat in these reactions.
self condensation
All carbonyl compounds that have one or more alpha hydrogens, on the carbons adjacent to the carbonyl group, run the risk of suffering a self-condensation reaction if the corresponding rigor is not followed.
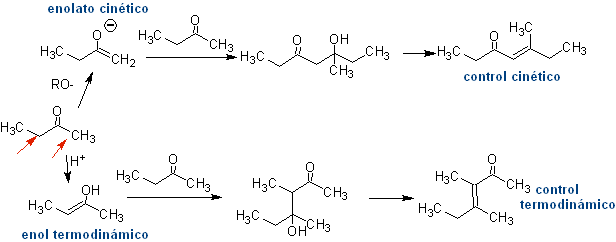
Thus, for example, if a non-hydroxylated base such as EtONa is added to a 2-butanone, an enolate will be formed that could eventually combine with another molecule of the same ketone. Similarly, if H+ is added, self-condensation can also occur, as can be seen in the following scheme: This can be minimized by adding at the same rate, from separate feeders, the base or acid to the compound that will provide the enol or enolate and the carbonyl compound that will be attacked on the carbonyl carbon. A three-necked flask and magnetic stirring can be used.
cross condensation
Ideally, in condensation reactions of carbonyl compounds, one of the reacting molecules should enolize rapidly, while the other should preferably have no Hα. , to ensure that no other by-products are formed
The retrosynthesis that is analyzed below is a good example of what was previously indicated.
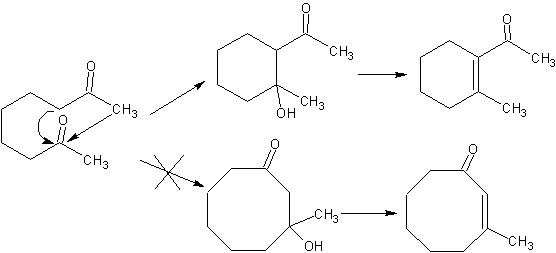
Intramolecular condensation .
When there are two functional groups in a molecule that directly affect the alpha carbons in relation to the carbonyl, it is most probable that there is an intramolecular self-condensation and the cycles that are formed must have high stability, as is the case of rings of five and six links. Rings with a greater number of difunctionalized links (7, 8 etc.), are not probable products due to the lability of these cycles.
Using the Mannich reaction.
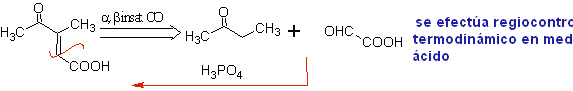
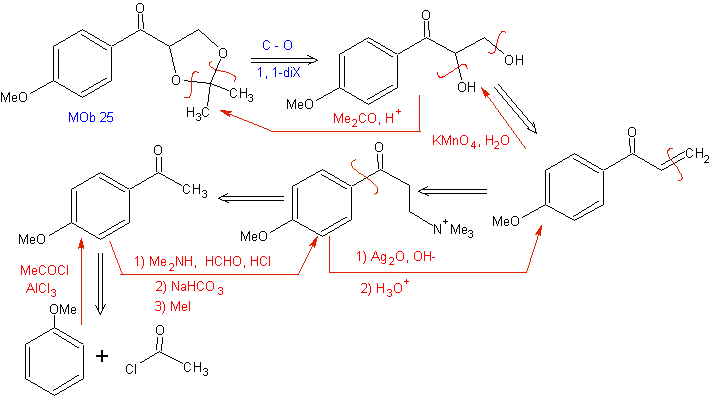
In conjugate addition reactions on alpha, beta-unsaturated carbonyl ( α, β-inssatCO) substrates, characteristic of the Michael reaction, the need to use vinyl ketones appears. But when it is tried to be prepared from the condensation of the ketone and the formaldehyde, other polymerization products of the aldehyde are formed that make its preparation difficult.
The Mannich reaction allows vinyl ketones to be obtained during the synthesis and at the necessary moment, which by other means presents serious difficulties in its preparation. This aspect is illustrated in the synthesis of MOb 25
Activation of the groups
System not activated:
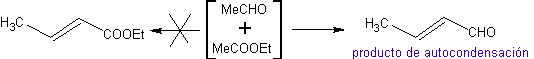
system activated
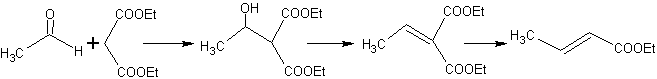
¨ enamines
It has already been said that one way to have an activated enolate is the formation of an enamine, between the carbonyl compound and a secondary amine. This aspect is shown in the following synthesis example.
Retrosynthetic analysis: In the synthesis of MOb 26, the proper use of enamines is observed to guarantee the formation of the compound 1,3 diCO.
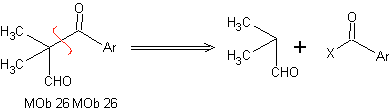
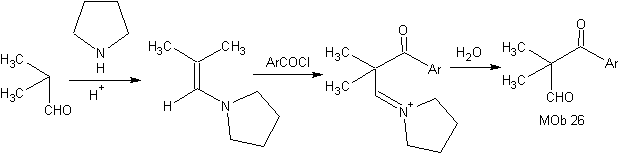
Synthesis: The enamine formed, allows its respective acylation, to reach the Mob26
¨ birch reduction
This reduction constitutes a good alternative to prepare cyclohexenones, as can be seen in the synthesis of MOb 27:
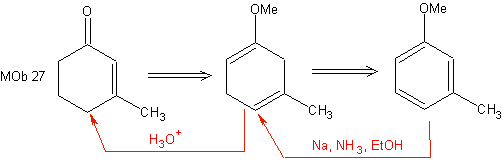
However, it should be remembered that certain α, β compounds -carbonyl unsaturated, can also be prepared by the Wittig reaction and through the Reformatsky reaction.
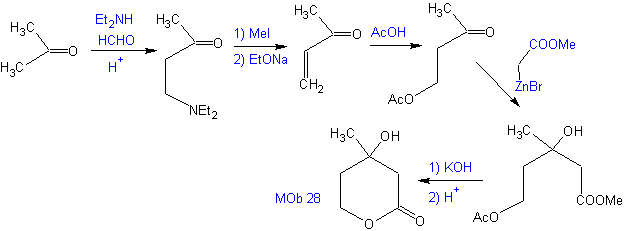
Propose a synthesis plan, from simple materials for MOb 28.
Retrosynthetic analysis: Initially the lactone is disconnected, followed by disconnection as a 1,3-diO pattern. The α,β-unsaturated carbonyl compound (α,β- insat. CO) generated could have been obtained by the Hoffmann elimination and ketoamine, by the Mannich reaction
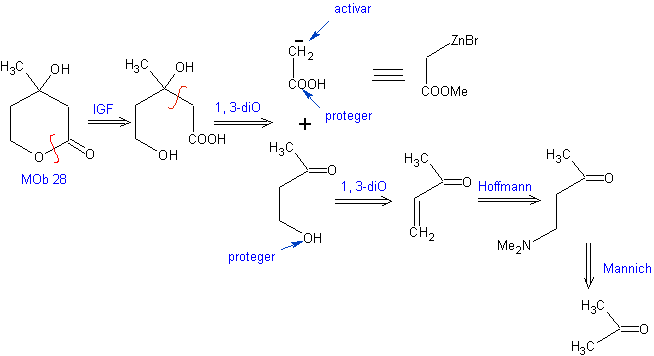
Synthesis: The synthesis of Mob 28, is very appropriate to show the control that must be exercised over certain reaction centers to achieve the desired and necessary transformations.