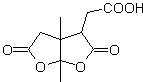
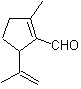
The 1,6-difunctionalized compounds preferably use the reconnection strategy for their respective synthesis; This strategy can very well be combined with the Diels-Alder reaction, which generally produces six-membered olefinic adducts, or the Birch reduction of benzene rings, which likewise generates six-membered olefinic products.
1.
1,6-dioxygenated compounds
The reaction that generates dicarbonyl compounds, of different possible combinations: diketones, ketoacids, ketoaldehydes, diacids, etc. and at different distances from one another, is undoubtedly the reaction of ozonolysis of olefinic compounds.
Depending on the structure of the substrate and the reaction conditions on the ozonide intermediate formed, an enormous diversity of compounds will be achieved as a result of the cleavage of the olefinic double bond. Of these, those that are in a 1, 6 – dioxygenated ratio are of special interest, as can be inferred from the following synthetic “reconnection” operation:
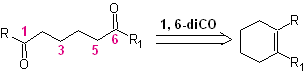
The best way to understand the operation of this "synthetic reconnection operation" is
will be achieved through the solution of the synthesis of the following organic molecules:
MOb 50
| MOb 51
| MOb 52
| ||
MOb 53
| MOb 54
| MOb 55
|
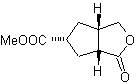
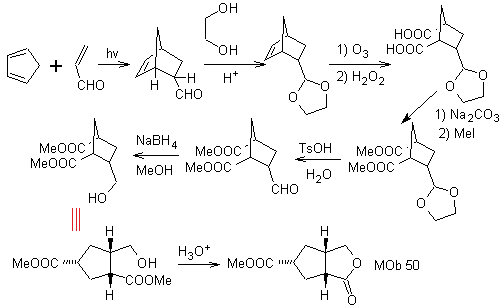
MOb 50 . Retrosynthetic analysis : In the first instance it is disconnected by the lactone function of the molecule. On the generated precursor molecule, in turn, it can be argued that its formation may have occurred from the diacarboxylic acid in position 1-6. Which are reconnected to give rise to the alkene that produced them by oxidative ozonolysis reaction. The alkene formed is a typical Diels-Alder adduct between cyclopentadiene and crotonaldehyde.
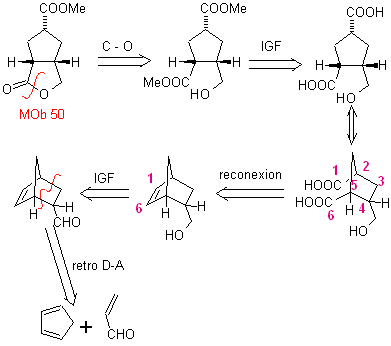
Synthesis: The Diels-Alder reaction between cyplopentadiene and the α,β-unsaturated aldehyde provides the alkene adduct, for its corresponding opening by oxidative ozonolysis, prior to a protection reaction of the aldehyde group, which is subsequently deprotected, to be reduced to the alcohol function. This alcohol reacts with the ester group in an acid medium to form the desired lactone, MOb 50
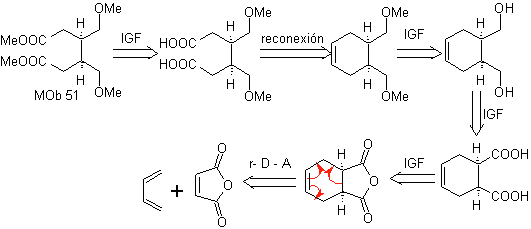
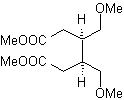
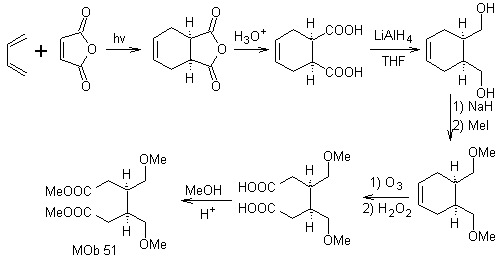
Synthesis. With the Diels-Alder reaction, the cyclic anhydride adduct is formed, which after being hydrolyzed is reduced to the respective alcohol, which by Williamson is transformed into ethers. The cyclohexene is opened by oxidative ozonolysis and the acid groups react in an acid medium with the methyl alcohol to transform into
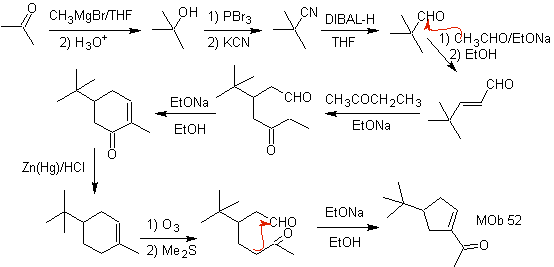
MOb 52 . Retrosynthetic analysis: Initially disconnected
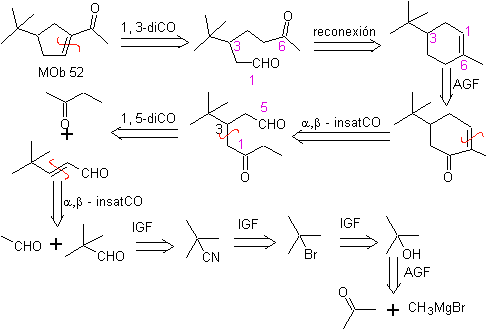
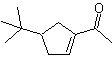
Synthesis : Acetone allows the formation of the intermediate t-Butylformaldehyde, which in a basic medium and ethanol condenses with ethanal. To the product, α, β-insat CO formed, the butanone enolate is added, the same basic medium allows intramolecular cyclization. Then the C=O is transformed into –CH2, by reduction. The cycloalkene produced is opened by ozonolysis in Me 2 S. And it is again cyclized in a basic medium to obtain
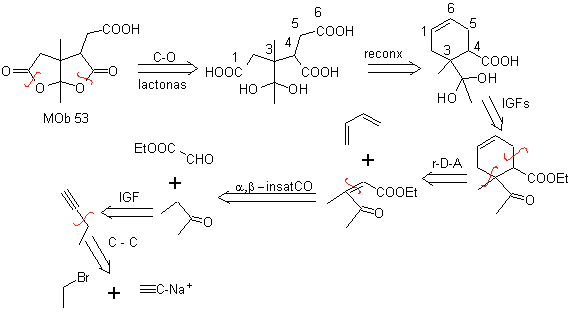
MOb 53 . Retrosynthetic analysis: It begins with the simultaneous disconnection of the lactones from
On the other hand, butanone is prepared from a terminal acetylene and the latter from an ethyl halide and sodium acetylene.
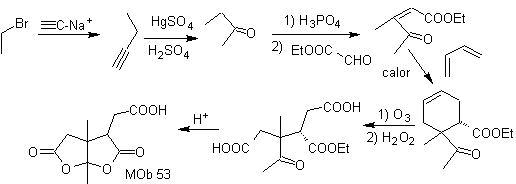
Synthesis. After the opening of the cyclohexene, by oxidative ozonolysis, The acid hydrolysis of the remaining ester group and the corresponding formation of the ketone hydrate is sufficient for the formation of the lactones and the respective closing of the cycle, to produce
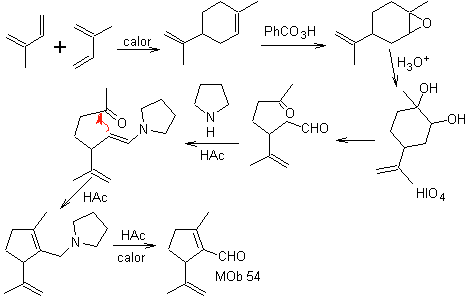
MOb 54 . Retrosynthetic analysis : The disconnection by the double bond of
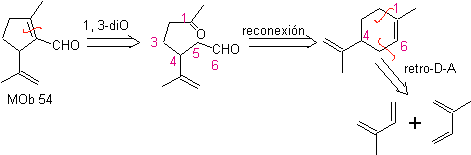
Synthesis: The Diels-Alder reaction between two molecules of 2-methyl butadiene forms an adduct that with HMCPBA forms an epoxide with the most reactive center.
·
On acid hydrolysis of the epoxide a diol is formed which is oxidized by acid. periodic, towards a 1,6-diCO compound. The CHO group is protected with an enamine, while activating its C alpha, for a condensation in an acid medium, the system is heated and we obtain
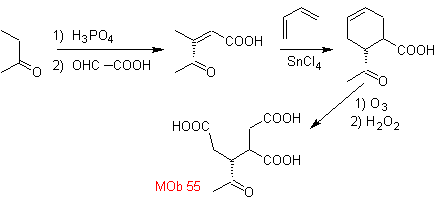
MOb 55. Retrosynthetic analysis : The synthesis of
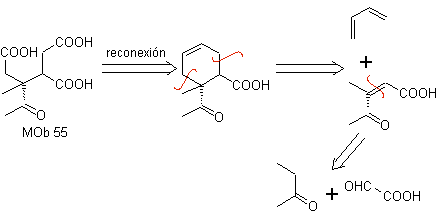
Synthesis. The reactions indicated have already been studied in the synthesis of