In this section we will see examples of cyclizations that take place through intermediates of radical type, carbene or nitrene.
A) We begin with a radical cyclization, which uses tributyltin hydride as a reagent and is initiated by AIBN
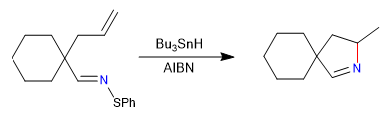
Mechanism:
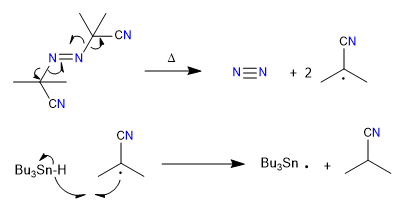
1. initiation
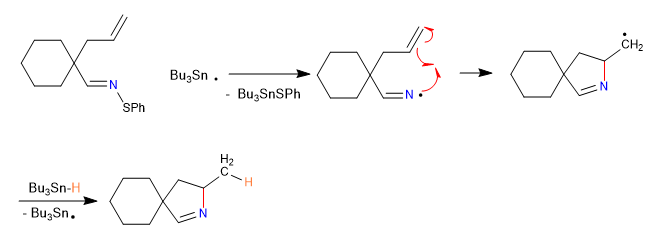
2. Propagation
B) In this second example we will take advantage of the reactivity of the nitrenes through the carbon-nitrogen insertion to obtain the carbazole.
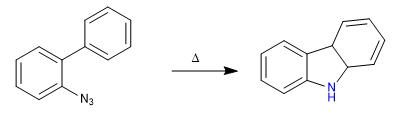
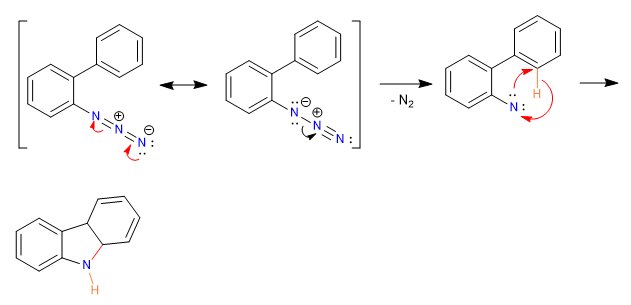
Mechanism:
C) Synthesis of indole from azide, via nitrene.
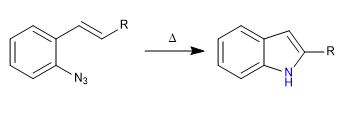
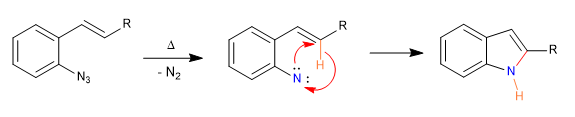
Mechanism:
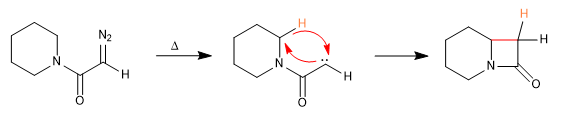
D) Cyclization processes via carbenes
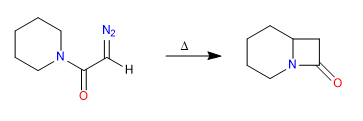
Mechanism: