NOE effect
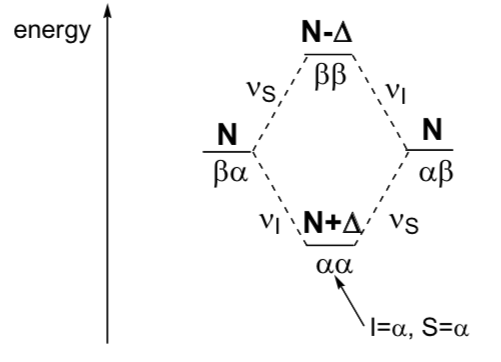
The nuclear Overhauser effect occurs when a proton, whose ratio of nuclei with spin +1/2 and –1/2 is altered (which is achieved with strong proton irradiation) interacts by dipole coupling with a neighboring proton, altering its ratio. of nuclei with spin +1/2 and –1/2 and thus changing the intensity of the absorption. The chemical shift is not altered. It is used to detect protons that are close to each other. It is very sensitive to distance because the dipole interaction depends on r -6 . The NOE effect only works at distances less than 5Å and the number of chemical bonds that separate the two protons does not matter.
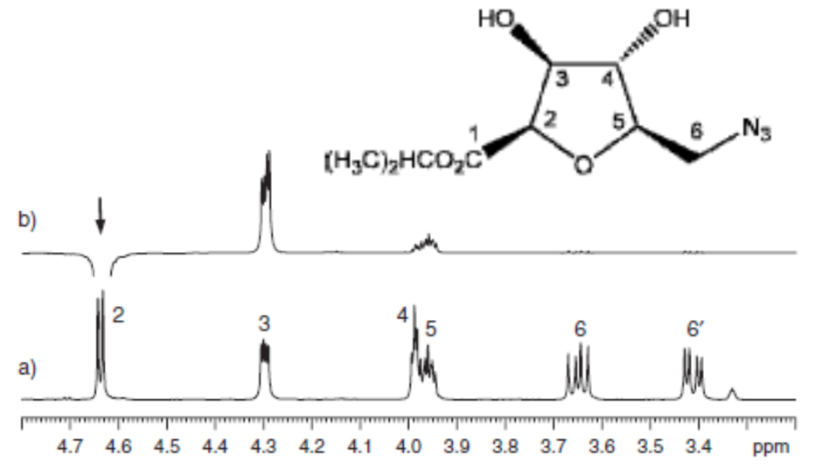
Figure 1. Example NOE
1- Where does the NOE come from?
Consider two nuclei, I and S, that share a dipole (through-space) coupling. These dipolar couplings depend on the relative orientation of I and S. In solution, molecular flipping averages these couplings, so they do not appear in typical NMR spectra. However, magnetization can still be transferred between them.
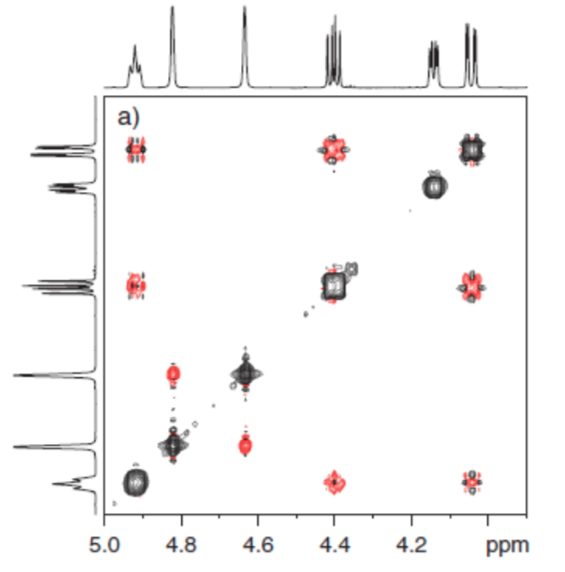
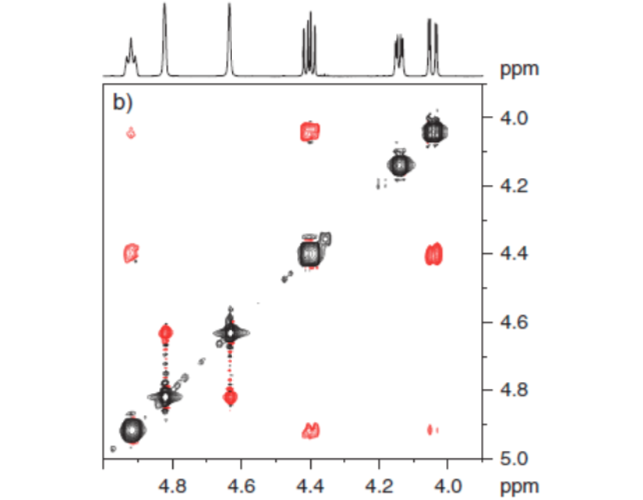
Figure 2. lys represented.
Now if we consider the effect of perturbing the equilibrium populations of spin S in some way on the intensity of the spin I signal. The NOE is defined as:
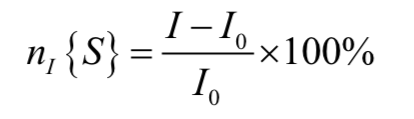
For simplicity, suppose that I and S do not share a scalar coupling (J). The simplest NOE experiment is the steady state experiment. One selectively saturates spin S, and then applies a 90° pulse to observe the effect this has on the spin population at spin I:
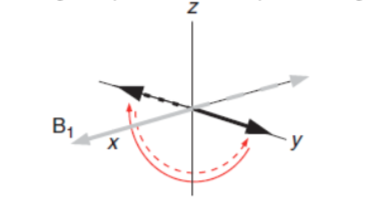
Figure 3. Pulse in NMR schematized at 90