Site-selective C−H difunctionalization of N-alkyl activated azaarenes via the synergistic catalysis of graphene oxide and visible light
Dehao Duan,a Haiping He,a Weiyi Ding,c Dongcheng Yi,a Yingzhen Lai,a Aiqiong Huang,a Jie Liu,b Wengle Wu,b Xiangjun Penga*
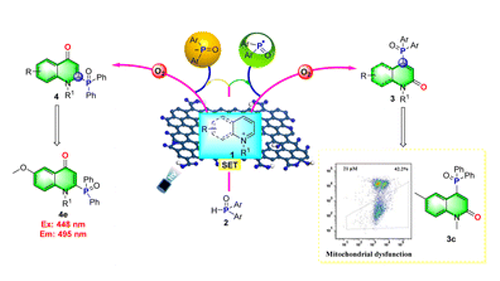
Strategies for site-selective C–H difunctionalization on N-alkyl activated azaarenes have attracted considerable attention as a powerful tool in heterocyclic chemistry. By employing graphene oxide (GO) as a heterogeneous cocatalyst, the visible light-induced site-selective difunctionalizations of pyridiniums/quinoliniums provided a distinct and straightforward synthetic route toward C4- and C2-selective phosphonation of the pyridinone/quinolinone/quinolone cores. Furthermore, the site-selectivity on quinoliniums could be successfully switched from C4 to C2 by changing the base and solvent. This coordination of catalysis systems drove the regioselective phosphonyl radical and phosphoryl anion addition to N-alkyl activated azaarenes, respectively, and subsequent oxidation with air as the terminal oxidant. In vitro antitumor studies showed that complex 3c participated in mitochondria-mediated pathways on the apoptosis of HeLa cells to 42.2% (21 μM). Phosphorus-based organocatalyst 4e, as one of the optical materials, offered efficient tuning of the emission and quantum yields.
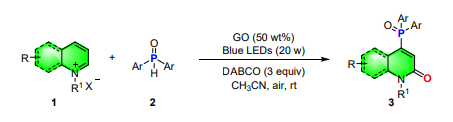
2.1 General procedures for synthesis of C4-phosphonated products
Halogenated pyridinium/quinolinium salts (1) (0.20 mmol), phosphine oxides (2) (0.24 mmol), DABCO (0.6 mmol), GO (50 wt%), were added to a 10-mL quartz tube in CH3CN (2 mL), and then the mixture was irradiated with blue LED strip (20 W) (approximately 1.5 cm away from the light source) at room temperature under air atmosphere. After complete conversion of the substrate (monitored by TLC), the reaction mixture was diluted with 20 mL of DCM, and the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 1:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired products 3.
2.2 General procedures for synthesis of C2-phosphonated quinolones
Halogenated quinolinium salts (1) (0.20 mmol), phosphine oxides (2) (0.24 mmol), NaOH (0.2 mmol), GO (50 wt%), were added to a 10-mL quartz tube in CH3OH (2 mL), and then the mixture was irradiated with blue LED strip (20 W) (approximately 1.5 cm away from the light source) at room temperature under air atmosphere. After complete conversion of the substrate (monitored by TLC), the reaction mixture was diluted with 20 mL of DCM, and the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 1:2). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the 2-phosphonated quinolones 4.
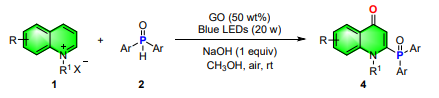
https://pubs.rsc.org/en/content/articlelanding/2023/qo/d3qo01375h
https://www.rsc.org/suppdata/d3/qo/d3qo01375h/d3qo01375h1.pdf