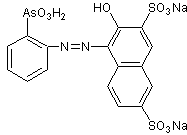
THORINE SYNTHESIS
The analytical applications of thorin, the working parameters, its limitations and prospects, are addressed and explained in sufficient detail in specialized chemical analysis publications such as Analytical Chemistry . A brief summary of them shows the following applications:
In an acid medium : it is used for the quantitative determination of the elements thorium (Th), zirconium (Zr), fluorine (F), hafnium (Hf) and uranium (U).
In a basic medium : The disulfonic acid form of thorine, better known as Thoron, is used in the quantitative determination of lithium (Li).
In a neutral medium : thorin is used as an indicator for the determination of sulfate ions (SO 4 2- ) in aqueous solutions.
Thorin is also used to determine SO 2 in air.
Research Problem.
Given the demand for this reagent for different analytical determinations and particularly for the colorimetric analysis of lithium in brines from the Gran Salar de Uyuni and the low supply of it in the national market, the need arises to synthesize thorine, from simple materials . and affordable in our environment.
With this purpose, the designed synthesis routes are approached from the RETROSYNTHESIS paradigm , being applicable the methods of The Synthesis Sheets, the Disconnection or Synthon Method and the Tree Method. of Synthesis.
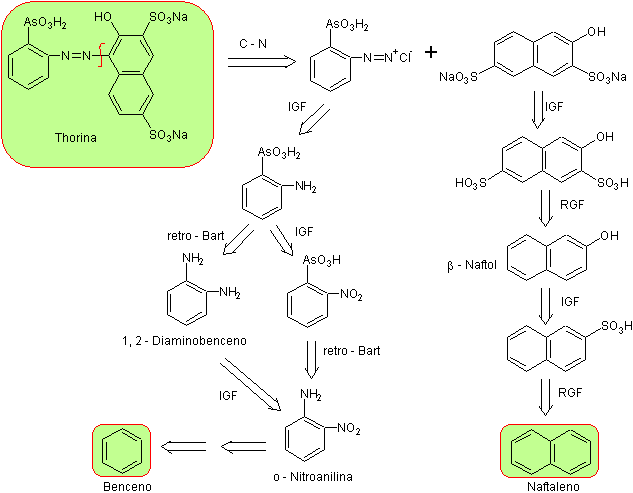
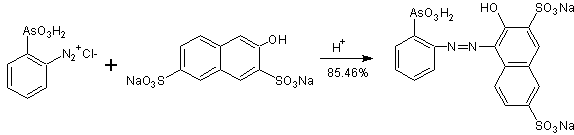
In relation to the synthesis of thorin , there is a brief and summarized description of the synthesis of thoron in the literature , according to the proposed direction by Kuznetsov , that is to say, it is indicated that for the preparation of thoron, it is required that the o-aminophenylarsonic acid be diazocopulated with the disodium salt of 2-naptol-3,6-disulfonic acid (salt R) in an acid medium.
Synthesis design for the Thorina .
The design is approached by the method of disconnections or Sintón, which contemplates two stages. The first is related to the retrosynthetic analysis and the second to the synthesis in the direction of what happens in the laboratory, that is, from the starting materials until arriving at the Target Molecule (MOb).
to) Items structural and reactivity to be considered, for the retrosynthetic analysis
Thorin is typically an azo compound, therefore a dye. The preparation of these compounds generally comprises two fundamental reactions, which are: diazoation and diazocoupling (or simply coupling).
On the other hand, all the coupling precursor molecules used for the formation of azo dyes have a common character, that is: an active hydrogen atom linked to a carbon atom.
The following are widely used as precursor molecules (substrates) for coupling: Compounds that have phenolic hydroxyls, such as phenols and naphthols, Aromatic amines, Molecules with enolizable aliphatic ketone groups, and Heterocyclic molecules such as pyrrole, indole, etc. .
In relation to the copulation reaction, certain heuristic principles must be taken into account, namely:
· Phenols couple more easily than amines and members of the naphthalene series more easily than benzene.
· Substituents with negative inductive effect (-I), such as halogens, nitro, sulfa, carboxyl, and carbonyl groups retard coupling.
· An alkyl or alkoxy group in the ortho or meta position with respect to an amino group promotes coupling. And if they are in positions 2 and 5 with respect to the amino group, they are especially good couplers.
· In the benzene series, coupling ordinarily occurs at the para position with respect to the hydroxyl (-OH) or amino (-NH 2 ) group. If the position for is occupied, the link occurs at the ortho position.
· In the naphthalene series, when the amino hydroxyl group is at position 2 ( b ), the reactant is coupled at position 1 ( a ). If the hydroxyl or amino group is in the alpha position, the bond usually occurs at the 4 position; but if position 3 or 6 is occupied by a sulfonic group, the union takes place in the beta position.
· When there are two possible positions of coupling, the position of the bond is frequently decided by the pH of the medium: The coupling occurs in ortho with the amino group when it is carried out in an acid medium and in ortho with the hydroxyl group when it is carried out in a basic medium. .
Based on the previously mentioned heuristic elements, there are various synthesis designs for thorin, which respond to retrosynthetic analysis . , proposed . Which in essence is a convergent synthesis, expressed by the diazo coupling of the disodium salt of 2-naphthol-3,6-disulfonic acid, commonly known as "R salt", with orthodiazophenylarzonic chloride in an acidic medium
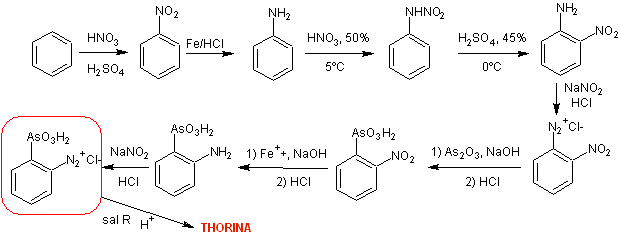
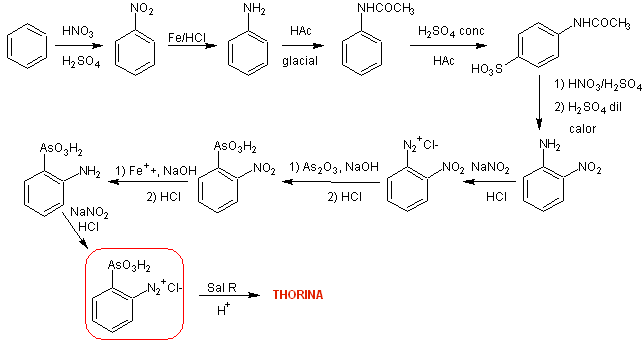
It can be seen in the retrosynthetic analysis that a key or strategic intermediate is ortho -nitroaniline, an aspect that leads to simple starting materials such as benzene and naphthalene respectively.
Source: self made
Orthonitroaniline , It can be synthesized by different procedures, with different levels of complexity and yields. Of the eleven experimentally investigated alternatives, only eight are indicated in the retrosynthesis rosette.
From it, disconnection alternatives 3 and 4 have been selected for this publication. , which in turn originate two convergent synthesis routes.
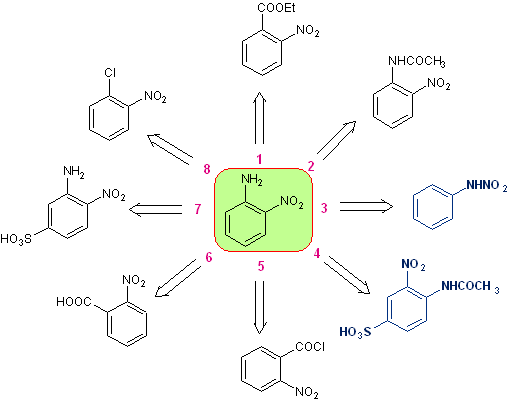
Retrosynthesis rosette for ortho-nitroaniline
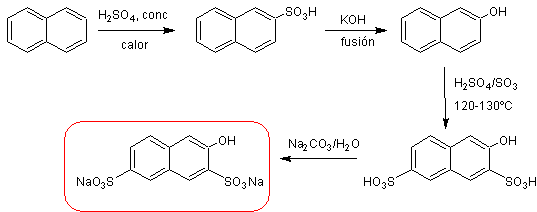
b) Synthesis of "salt R".
c) Synthesis of thorin.
Synthesis Route I : The strategy takes into account the formation of ortho -nitroaniline from Nitroamine rearrangement of nitraniline, in an acid medium.
Synthesis Route II. The strategy involves the formation of ortho -nitroaniline, through processes of protection and deprotection of the para position of the aniline.
Results
The experiments developed for each stage in the different Synthesis Routes have responded to a 2 2 factorial experimental design that allowed us to investigate the main effect of the factors and the interaction between them. , to optimize working conditions.
ü Experimental results of the preparation of “salt R”.
naphthalene ![]() sodium β-naphthalene sulfonate
sodium β-naphthalene sulfonate ![]() β-Naphthol
β-Naphthol ![]() “salt R”
“salt R”
r 1 = 87.89% r2 = 86.55% r3 =75.23%
The sulfonation yield of β -Naphthol is relatively low, because at the same sulfonation temperature, other products such as Schaeffer's salt (sodium 2-naphthol-6-sulfonate) and small amounts of the sodium salt of 2-naphthol-6,8-disulfonic acid (acid G), whose sodium salt is more soluble than the "R salt", an aspect that is used in the recrystallization process of the "R salt".
Overall yield : R salt R = (r 1 *r 2 *r 3 )*100
= (0.8789*0.8655*0.7523)*100 = (0.5723)*100 = 57.23%
ü Experimental results for Synthesis Route I.
The process that starts from benzene until arriving at ortho -diazophenylarzonic chloride (Cl-o-DFA), consists of eight stages and reaches a yield of R Cl-o-DFA = 23.39%.
On the other hand, the reaction in an acid medium, of ortho -diazophenylarzonic chloride with the "salt R", prepared according to the previously indicated schemes, to obtain THORINA, presents a yield of 85.46% .
Based on these experimental results, the total yield (Q TSI ) of Synthesis I, with a confidence limit of 95% is:
QTSI = [(57.23% + 23.39%)/2]*85.46 = 34.45%
The intrinsic cost of production for thorine, in the Synthesis I route is 0.81 $us/g
ü Experimental results for Synthesis Route II.
In this new synthesis alternative, benzene is transformed into ortho -diazophenylarzonic chloride in nine stages, with a yield RCl-o-DFA = 28.04%.
The reaction of this salt, ortho -diazophenylarzonic chloride, with the R" salt in an acid medium, as indicated above, reaches a yield of 85.46%, for which the total yield of Synthesis Route II (Q TSII ), is 36.44%.
The intrinsic cost of thorin production, according to this synthesis route, is 1.17 $us/g
conclusions
The different synthesis routes proposed and tested have the virtue of demonstrating that organic synthesis, the heart of organic chemistry, is an eminently predictive discipline, and is a highly valuable pedagogical tool.
Retrosynthetic analysis, expressed by the disconnection methodology in the design plane and the use of factorial experimental designs used in practice, are a powerful combination to face organic synthesis works of considerable depth levels.
The reproducibility of the lithium analysis in aqueous solutions in a basic medium, the stability of the lithium-thorine complex, the percentage of Na and As present in the molecule, as well as the IR and NMR spectra of the product obtained, demonstrate the high degree of purity of synthesized thorin.
BIBLIOGRAPHY
01. ALDRICH. Catalog Handbook of Fine Chemical . Ed. Aldrich Chemical Company Inc. 1994 – 1995.
02. CAICEO AF Experimental Design . S. ed. sf
03. CHARLOT G. Colorimetric Determination of Elements (principles and methods) Ed. Elsever Publishing Company, 1964
04. DOMÍNGUEZ XA Experimental Organic Chemistry . Ed. Limusa SA 1982
05. GOULD ES Mechanism and Structures in Organic Chemistry . Ed. Kapeluz. 1967
06. KIRK AND VARIOUS. Chemical Technology Encyclopedia . Spanish-American Ed., 19959
07. MILLER JC Statistics for Analytical Chemistry . Ed. Addison - Wesley Iberoamericana, SA Second Edition 1993.
08. THOMASON P.F. Anal Chem 28 (1956)
09. WARREN S. Organic Synthesis Design (Introduction to the Synthon method) . Ed. Alhambra, 1983.
Wilbert Rivera Munoz. This email address is being protected from spambots. You need JavaScript enabled to view it.