phenanthrene synthesis
(Synthesis Tree Method)
Phenanthrene is a polycyclic aromatic hydrocarbon, which contains three fused benzene rings, which is why it is an isomer of anthracene.
|
|
either |
|
The traditional synthesis methods of phenanthrene, those that involve the formation of cycles and their subsequent "aromatization", are linked to those proposed by Haworth and Bardhan - Sengupta (1932), as will be seen below.
It is also possible to propose other new methods, for Phenanthrene and in general for polycyclic aromatic compounds, based on the appropriate use of the Ullman coupling reactions, the Heck reaction, the Suzuki reaction and the MacMurry reaction, as well as the variants and extensions that present these reactions.
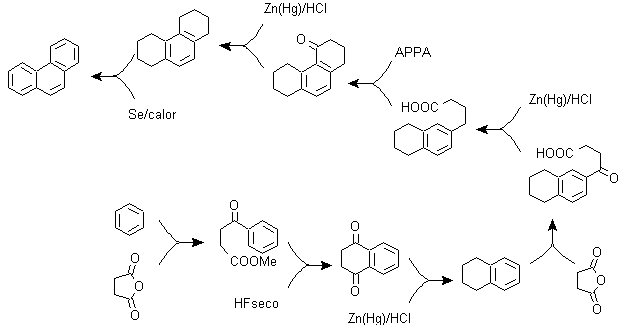
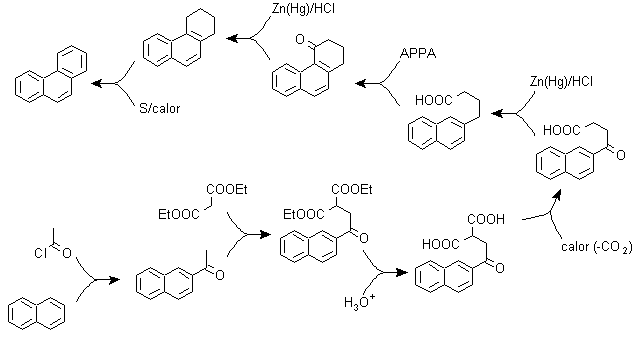
a) Haworth's synthesis:
This method is based on the Friedel-Crafts acylation reaction and has the drawback that the final cyclization to form the third ring fused to naphthalene is not selective, because it is also possible that the closure occurs in the other carbon adjacent to the group that contains the carboxylic function and thus forms an isomer, which due to the reaction conditions turns out to be the minority.
Method A :
The starting materials are usually naphthalene and succinic anhydride. To ensure that the acylation reaction with the anhydride occurs at carbon 2 (beta) of naphthalene, it is necessary to carry out the reaction at a temperature higher than 60 ºC.
At room temperature, the acylation position will be carbon 1 (alpha), which gives rise to a variant to the method, which, however, are essentially the same reactions that occur and there is also the formation of another isomer that is much less significant than in the first case. Therefore, it is preferably used as the official reaction for the preparation of phenanthrene by the Howorth method.
Method B :
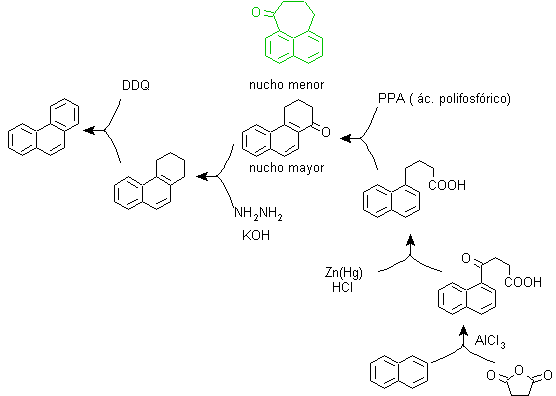
b) Bardhan–Sengupta synthesis (1932).
In this synthesis, the strategy goes through building or "assembling" the intermediate cycle that is then reduced (aromatized) by metallic selenium and heat, the cyclization is carried out by the reaction of an alcohol, on a benzene ring, catalyzed by phosphorus pentoxide, which at the same time abstracts the hydrogens from cyclohexanol. The cyclization turns out to be regiospecific, it is not carried out by the traditional Friedel-Crafts alkylation, which is why the formation of other isomers does not occur.
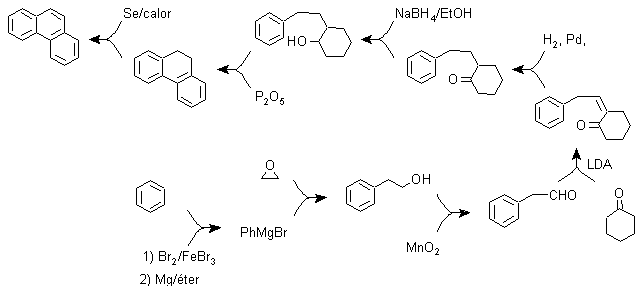
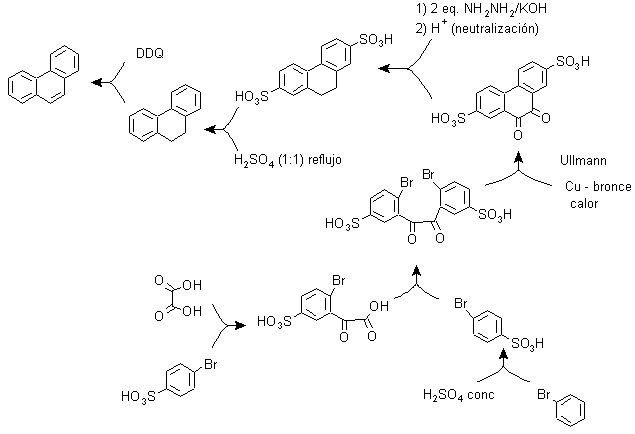
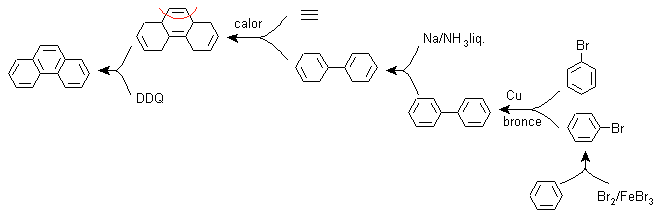
c) Synthesis, based on the strategy of the Ullman reaction .
Aryl groups can be coupled with good yields according to the Ullman reaction and some of its variants. In the synthesis that is proposed below, the internal cycle is formed by a coupling of two benzenes that have a halogen (bromine) substituent in their structure. The aromatization of this cycle is carried out through the action of a disubstituted quinone such as DDQ.
Due to this strategy, a group is located in the para position, to bromo benzene, which must temporarily occupy said position and whose removal from the benzene ring is later relatively easy, which occurs with the sulfonic group.
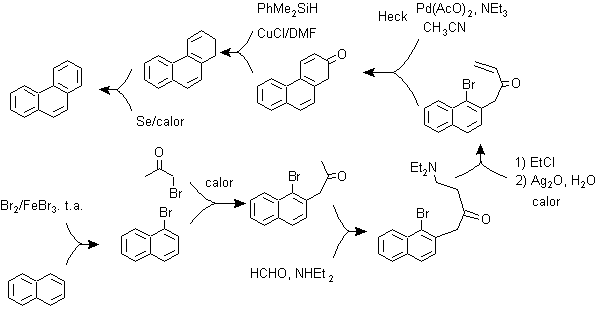
d) Synthesis, based on the Heck reaction strategy
The coupling from aromatic halides to alkenes, due to the catalytic action of Pd or its salts, can be another of the reactions used in a synthesis strategy, for fused polyaryls.
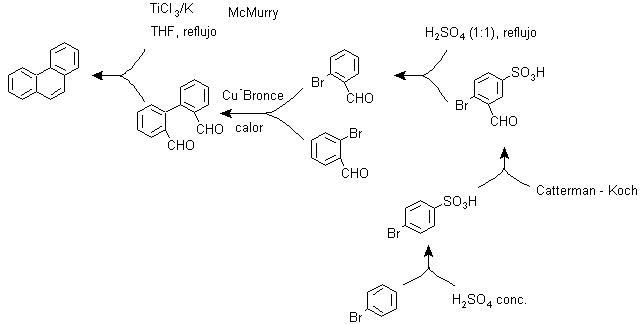
e) Synthesis, based on the MacMurry reaction strategy
The bimolecular reduction of aldehydes and ketones, catalyzed by Ti(III) or Ti(IV) salts, known as the MacMurry reaction, can also be used in a strategy for the synthesis of condensed polycyclic compounds, such as Phenanthrene and as can be seen below:
F) Synthesis, based on the strategy of the Diels-Alder reaction
The functionalization of phenanthrene to a non-aromatic tricyclic compound allows the formation of a precursor molecule of the adduct type, where the structure of the diene and the dienophile can be glimpsed, which allows the disconnection related to the Diels-Alder reaction to be applied. This precursor molecule can be obtained through the Birch reduction of biphenyl.
Biphenyl is the result of the coupling reaction of two moles of bromobenzene, according to Ullman.
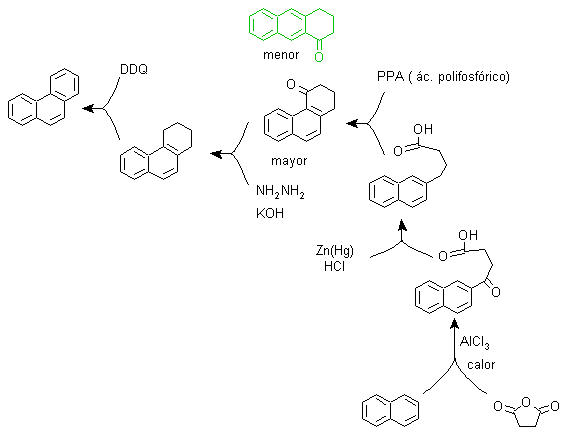
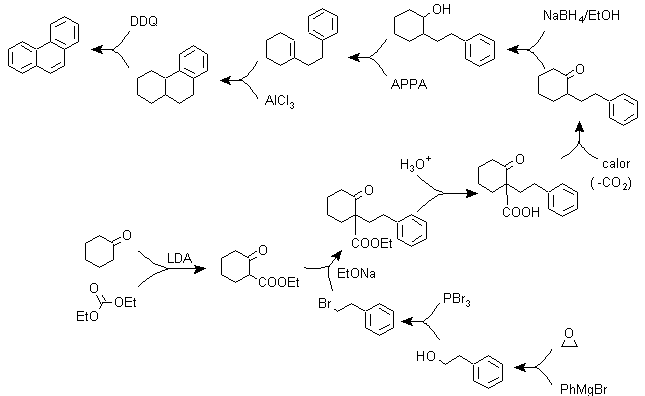
g) Variants of the synthesis based on Friedel-Crafts acylations and alkylations .
Syntheses that do not require special comments, because the reactions used are abundantly known.
g1.
g2.
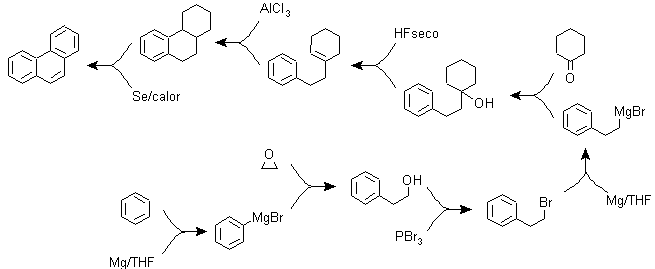
g3.
g4.