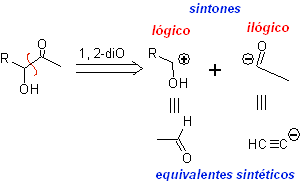
α-Hydroxycarbonyl compounds are disconnected at the CC bond that joins the two functions. This operation leads to a natural or logical synthon (the cationic synthon) and to an unnatural or illogical synthon (the anionic synthon). The synthetic equivalents can be aldehydes and acetylide ion respectively.
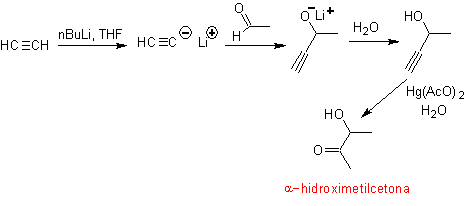
The terminal acetylene group with mercuric salts in an acid medium forms a methyl ketone. This reaction is also useful if the acetylene group is internal and also symmetric.
A special case of α-hydroxyketones are benzoins or diarylhydroxyketones , where the two R groups are aromatic or heterocycles. Benzoins are the result of the self-condensation of benzaldehyde catalyzed by cyanide ions.
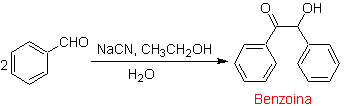
The benzoins may not be symmetrical, for example one of the aldehydes may be a pyridine aldehyde. The cyanide ion does not catalyze aliphatic aldehydes, which undergo the same coupling in the presence of thiazolium salts.
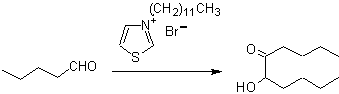
Aliphatic α-hydroxyketones can be formed from the condensation of carboxylic esters with metallic sodium in an inert solvent and under reflux. These hydroxyketones are called acyloins , and condensation reactions can occur intramolecularly and intermolecularly.
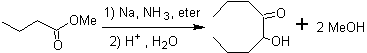
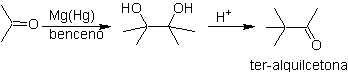
The compounds grouped together under the name of oxocarboxylic acids , which include aldehydo acids and ketoacids, are of great importance in the aliphatic series and both because of their biochemical relationship with the oxacids, and because of the synthesis reactions that can take place from them or from them. Its derivatives constitute an important group of organic compounds, on which intense work is being done in recent times.
2.- Compounds 1,2-Diols The 1,2-diols have in the olefins the best precursors for their preparation, it will only depend on the stereochemistry of the diol in question, to resort to one of the specific reactions summarized in the attached table.
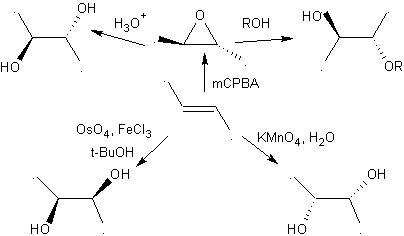
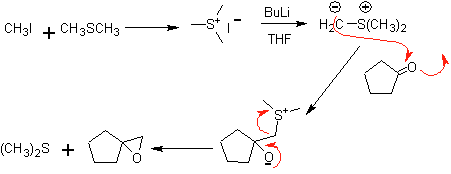
1,2-dioxygenated molecules can also be built using illogical electrophiles; The most important reagents of this type are the alpha-halogenated carbonyl compounds obtained by halogenation of the enol form of a carbonyl compound.
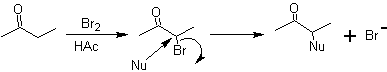
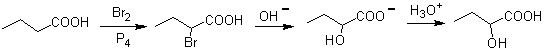
Another method that produces 1,2-difunctionalized compounds consists of the oxidative cleavage of double bonds by ozone to generate two carbonyls, which will vary according to the reaction conditions.
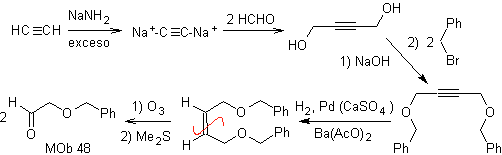
| Mob 48:
|
Retrosynthetic analysis. the MOb 48 is an unsymmetrical olefinic ether and with the group aldehyde at one end, which is why it can be assumed that it is the result of the opening of a double bond by ozonolysis followed by the reaction with Me 2 S. The double bond of the precursor is functionalized to a symmetric ethereal internal alkyne.
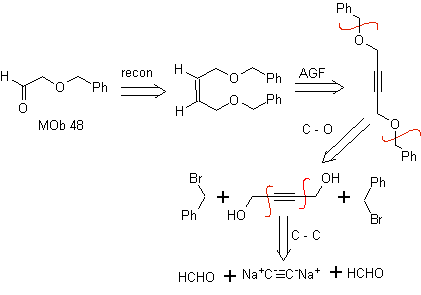
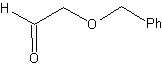
The ethers are the result of the application of the Williamson synthesis and the diol of the diacetylide reaction on aldehydes, as starting material.
synthesis . The diacetylide is made with an excess of sodamide, which acts without formaldehyde, to give the alcohol, which is etherified by Williamson. The alkyne is reduced to an alkene which is opened by ozonolysis, followed by reaction with Me 2 S to form the aldehyde.
synthesis . The diacetylide is made with an excess of sodamide, which acts without formaldehyde, to give the alcohol, which is etherified by Williamson. The alkyne is reduced to an alkene which is opened by ozonolysis, followed by reaction with Me 2 S to form the aldehyde.