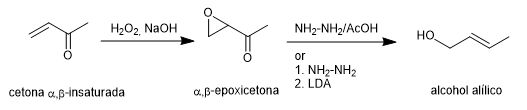
The Wharton synthesis allows α,β-epoxyketones to be transformed into allylic alcohols by treatment with hydrazine in acetic acid medium or hydrazine hydrate followed by strong base. The $\alpha,\beta$-epoxyketone is obtained from the $\alpha,\beta$-unsaturated ketone by oxidation with hydrogen peroxide in a basic medium.
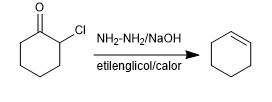
Starting with substituted α-ketones (haloketones, hydroxyketones, aminoketones), olefins are obtained by treatment with hydrazine in a basic medium under heating.
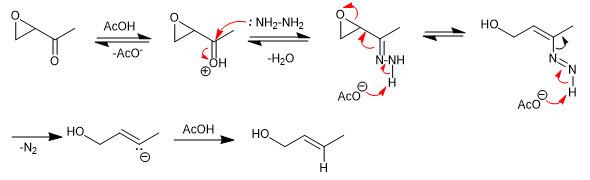
Mechanism:
The mechanism of the Wharton rearrangement is very similar to that of the Wolff-Kishner reaction. It begins with protonation of the carbonyl, followed by attack by hydrazine to form a hydrazone, which on deprotonation releases nitrogen with formation of a vinyl anion. Protonation of the vinyl anion yields the final product.