When studying the optical rotation of natural glyceraldehyde, it was observed that it coincided with the dextrorotatory enantiomer and it was named D-Glyceraldehyde. The levorotatory enantiomer, not present in nature, was named L-glyceraldehyde.
The D/L notation divides sugars into two enantiomeric families, although it should be noted that not all members of the D family are right-handed, nor are all members of the L family left-handed. However, the D/L notation distinguishes natural sugars from artificial ones. To recognize if a sugar belongs to the D or L series, we must look at the last chiral center of the chain, in D sugars this center has the notation R and in L the notation S. When the sugar is drawn in a Fischer projection, situation very common, we only have to look at the position of the -OH group in this last chiral center, if the -OH is located on the right it is a sugar belonging to the D series, if it is on the left the sugar belongs to the series L.
Let's see an example:
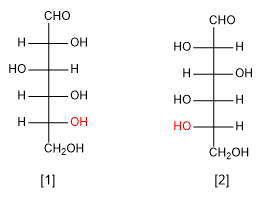
D-glucose ( -OH on the right)
L-glucose ( -OH on the left)
Note that the D and L sugars are enantiomers and have all chiral centers switched.
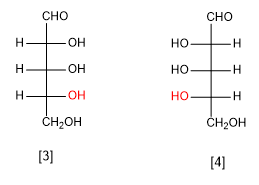
D-Ribose
L-Ribose
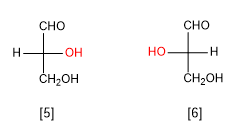
D-Glyceraldehyde
L-Glyceraldehyde.









