DIELS–ALDER THEORY
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 32183
- Details
- Germán Fernández
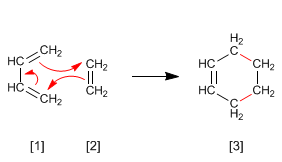
- DIELS–ALDER THEORY
- Hits: 37255
Diels-Alder is a stereospecific reaction, it produces a single diastereoisomer. To draw the stereoisomer formed in a Diels-Alder, three rules are followed:
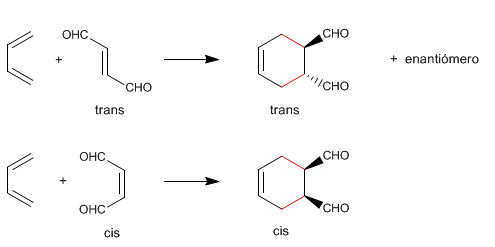
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 37407
Diels-Alder is a stereospecific reaction, it produces a single diastereoisomer. To draw the stereoisomer formed in a Diels-Alder, three rules are followed:

- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 19842
Alkynes can act as dienophiles in the Diels-Alder reaction. The reaction between diene and alkyne forms a 1,4-cyclohexadiene.
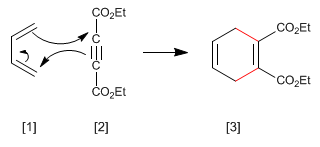
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 23156
The reverse reaction to Diels-Alder is called retro-Diels-Alder. Diels-Alder adducts decompose on heating into the diene and dienophile that formed them.
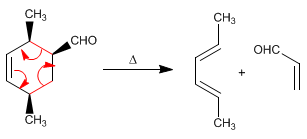
Read more: Inverse reaction to Diels-Alder (Retro-Diels-Alder)
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 1918
The intramolecular Diels Alder reaction occurs when the diene and the dienophile belong to the same molecule.
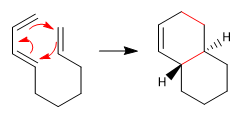
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 1874
Alkenes in the presence of light undergo cycloaddition reactions to form four-membered rings. These reactions are called [2 + 2] cycloadditions.
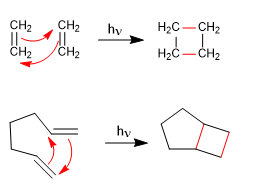
- Details
- Germán Fernández
- DIELS–ALDER THEORY
- Hits: 1921
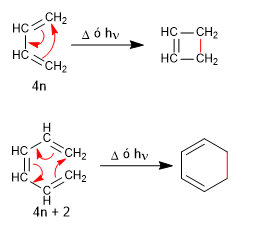
Both 1,3-butadiene (4n) and 1,3,5-hexatriene (4n + 2) cyclize with light or heat to give cyclobutene and 1,3-cyclohexadiene, respectively.
Like Diels-Alder, electrocyclic reactions are concerted and stereospecific.









