Draw the formulas of the following compounds:
| 1) 2-Methylhexa-1,5-diene 2) 2,3,4-Trimethylocta-1,4,6-triene 3) 4-tert-Butyl-2-chlorohept-1-ene 4) 3-Ethyl-2,4-dimethylhept-3-ene 5) 3,4-Diisopropyl-2,5-dimethylhex-3-ene 6) Hept-1-ene 7) cis-Oct-3-ene 8) 3-ethylpent-2-ene |
9) trans-1,4-Dibromobut-2-ene 10) 3-Chlorohex-2-ene 11) Buta-1,3-diene 12) Hexa-1,4-diene 13) 5-Methyl-3-propylocta-1,4,6-triene 14) 6-Methyl-6-propylnone-2,4,7-triene 15) 2,3,5-Trimethylocta-1,4-diene 16) 3-Propylhepta-1,5-diene. |
SOLUTION:
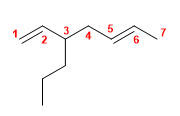
1) 2-Methylhexa-1,5-diene
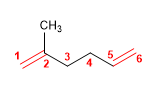
2) 2,3,4-Trimethylocta-1,4,6-triene
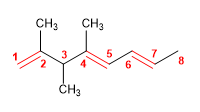
3) 4-tert-Butyl-2-chlorohept-1-ene
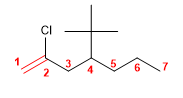
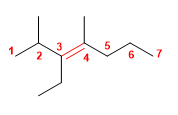
4) 3-Ethyl-2,4-dimethylhept-3-ene
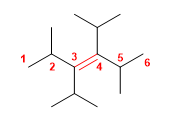
5) 3,4-Diisopropyl-2,5-dimethylhex-3-ene
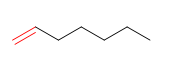
6) Hept-1-ene
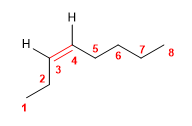
7) cis-Oct-3-ene
8) 3-ethylpent-2-ene 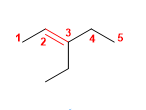
![]() The cis notation indicates that the equal groups (hydrogens) of the 3,4 positions are oriented to the same side of the alkene.
The cis notation indicates that the equal groups (hydrogens) of the 3,4 positions are oriented to the same side of the alkene.
9) trans-1,4-Dibromobut-2-ene
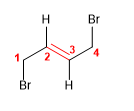
10) 3-Chlorohex-2-ene
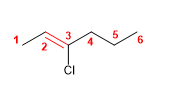
![]() The trans notation indicates that the equal groups of the double bond (hydrogens) are oriented to opposite sides.
The trans notation indicates that the equal groups of the double bond (hydrogens) are oriented to opposite sides.
11) Buta-1,3-diene
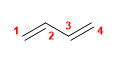
12) Hexa-1,4-diene
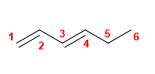
13) 5-Methyl-3-propylocta-1,4,6-triene
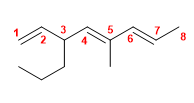
14) 6-Methyl-6-propylnone-2,4,7-triene
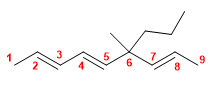
15) 2,3,5-Trimethylocta-1,4-diene
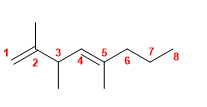
16) 3-Propylhepta-1,5-diene.