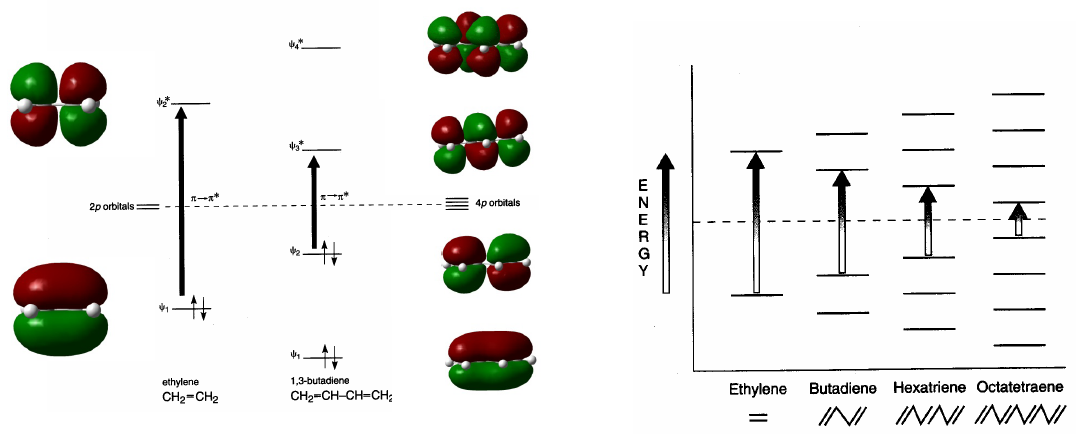
Conjugated systems absorb at longer wavelengths than unconjugated ones. As the conjugation increases, the energy difference between HOMO and LUMO decreases and the radiation necessary to produce the transition $\pi \rightarrow \pi^{\ast}$ decreases its wavelength.
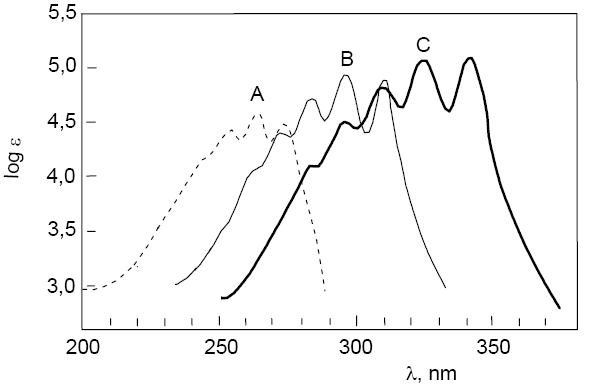
Next, the spectra of three polyenes with formula $CH_3-[CH=CH]_n-CH_3$ where n=3 in A are superimposed; n=4 in B; n=5 in C. The shift of the vis-UV spectrum towards longer wavelengths can be observed as well as a slight increase in the $\epsilon_{max}$
Substances with a high number of conjugated multiple bonds come to absorb in the visible region. Beta-Carotene absorbs in the blue region $\lambda_{max}=452\;nm$ and shows orange color (reflected wavelength or complementary color).